QUESTION 1 What is the approximate value of the equilibrium constant, K n, for the neutralization of nitrous acid with ammonia, shown in the equation below? The Ka for HNO 2 is 4.5 x 10 -4 and the Kb for NH 3 is 1.8 x 10 5. HNO 2( aq) + NH 3( aq) NH 4NO 2( aq) O 8.1 x 1019 8.1 x 105 4.5 x 1010 1.8 x 109 QUESTION 2 1. The following pictures represent solutions of CaCO a which mav also contain ions other than Ca 2+ and CO a 2- which are not shown. Grav Click Save and Submit to save and submit. Click Save All Answers to save all ansuwers. Save All Answers MacBook Air F10 吕0 F8 F9 000 F7 F6 F5 F3 F4 & 8. 「会 云 %23
QUESTION 1 What is the approximate value of the equilibrium constant, K n, for the neutralization of nitrous acid with ammonia, shown in the equation below? The Ka for HNO 2 is 4.5 x 10 -4 and the Kb for NH 3 is 1.8 x 10 5. HNO 2( aq) + NH 3( aq) NH 4NO 2( aq) O 8.1 x 1019 8.1 x 105 4.5 x 1010 1.8 x 109 QUESTION 2 1. The following pictures represent solutions of CaCO a which mav also contain ions other than Ca 2+ and CO a 2- which are not shown. Grav Click Save and Submit to save and submit. Click Save All Answers to save all ansuwers. Save All Answers MacBook Air F10 吕0 F8 F9 000 F7 F6 F5 F3 F4 & 8. 「会 云 %23
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 91GQ: m-Nitrophenol, a weak acid, can be used as a pH indicator because it is yellow at pH above 8.6 and...
Related questions
Question
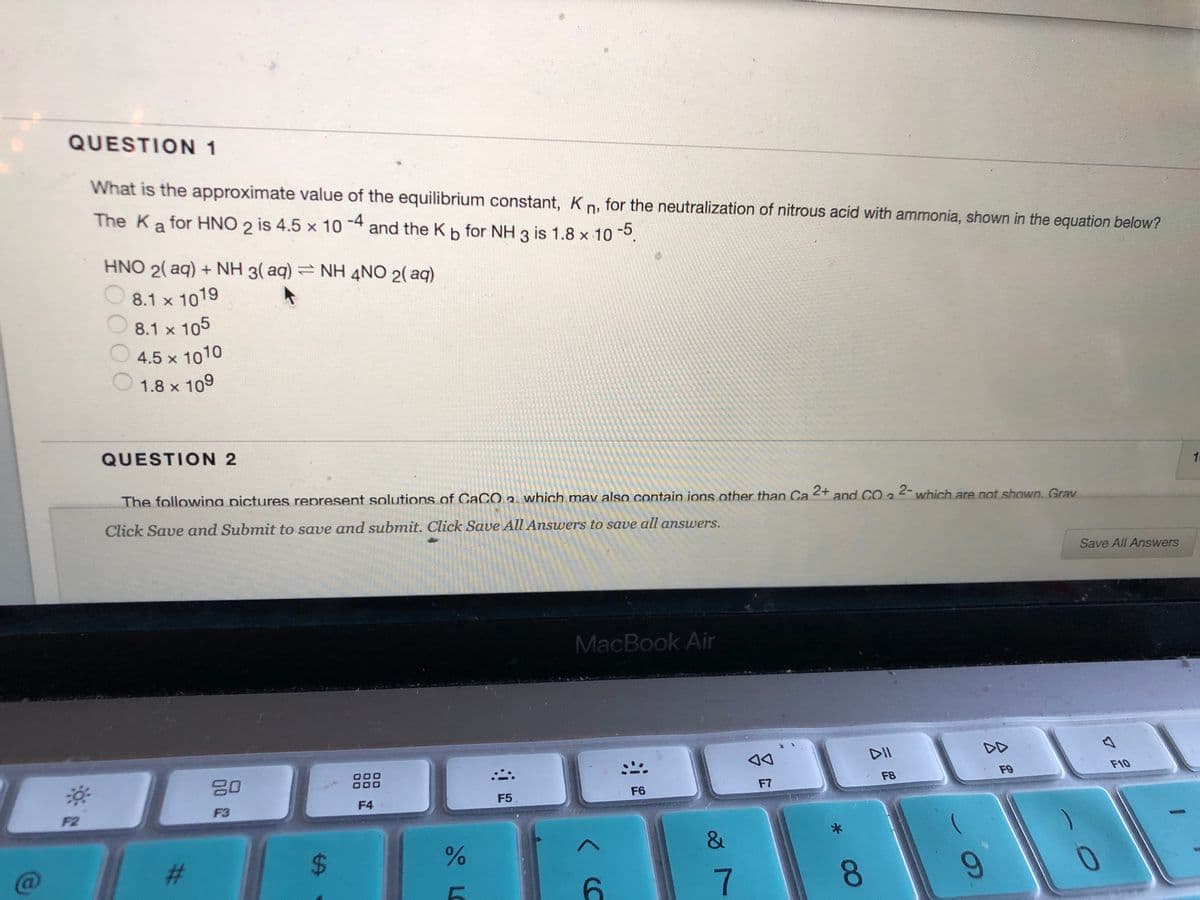
Transcribed Image Text:QUESTION 1
What is the approximate value of the equilibrium constant, K n, for the neutralization of nitrous acid with ammonia, shown in the equation below?
-4
The Ka for HNO 2 is 4.5 x 10
and the Kb for NH 3 is 1.8 x 10.
HNO 2( aq) + NH 3( aq) = NH 4NO 2( aq)
8.1 x 1019
8.1 x 105
4.5 x 1010
1.8 x 109
QUESTION 2
The followina pictures represent solutions of CaCO2. which mav also contain ions other than Ca
2+
and CO 3
2- which are not shown. Grav
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Save All Answers
MacBook Air
DII
DD
20
F10
F8
F9
F6
F7
F4
F5
F2
F3
23
7
8.
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning