QUESTION 27 In a 1.00 M aqueous solution of sulfuric acid, H₂SO4, what is the sulfate (SO42) concentration at equilibrium? 1. H₂SO4 (aq) + H₂0 (1) H30* (aq) + HSO4 (aq) 2. HSO4 (aq) + H₂O (1) <> H3O+ (aq) + SO4²- (aq) 3.2 H₂0 (aq) <> H30* (aq) + OH¯ (aq) O 0.500 M O 0.109 M O 1.00 M O 0.012 M QUESTION 28 L Ka2 = 0.012 Kw=1.0 x 10-14 Check all that is true for a buffer solution. The pH of a buffer solution can be determined by the Henderson-Hasselbalch equation Click Save and Submit to save and submit. Click Save All Answers to save all answers.
QUESTION 27 In a 1.00 M aqueous solution of sulfuric acid, H₂SO4, what is the sulfate (SO42) concentration at equilibrium? 1. H₂SO4 (aq) + H₂0 (1) H30* (aq) + HSO4 (aq) 2. HSO4 (aq) + H₂O (1) <> H3O+ (aq) + SO4²- (aq) 3.2 H₂0 (aq) <> H30* (aq) + OH¯ (aq) O 0.500 M O 0.109 M O 1.00 M O 0.012 M QUESTION 28 L Ka2 = 0.012 Kw=1.0 x 10-14 Check all that is true for a buffer solution. The pH of a buffer solution can be determined by the Henderson-Hasselbalch equation Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
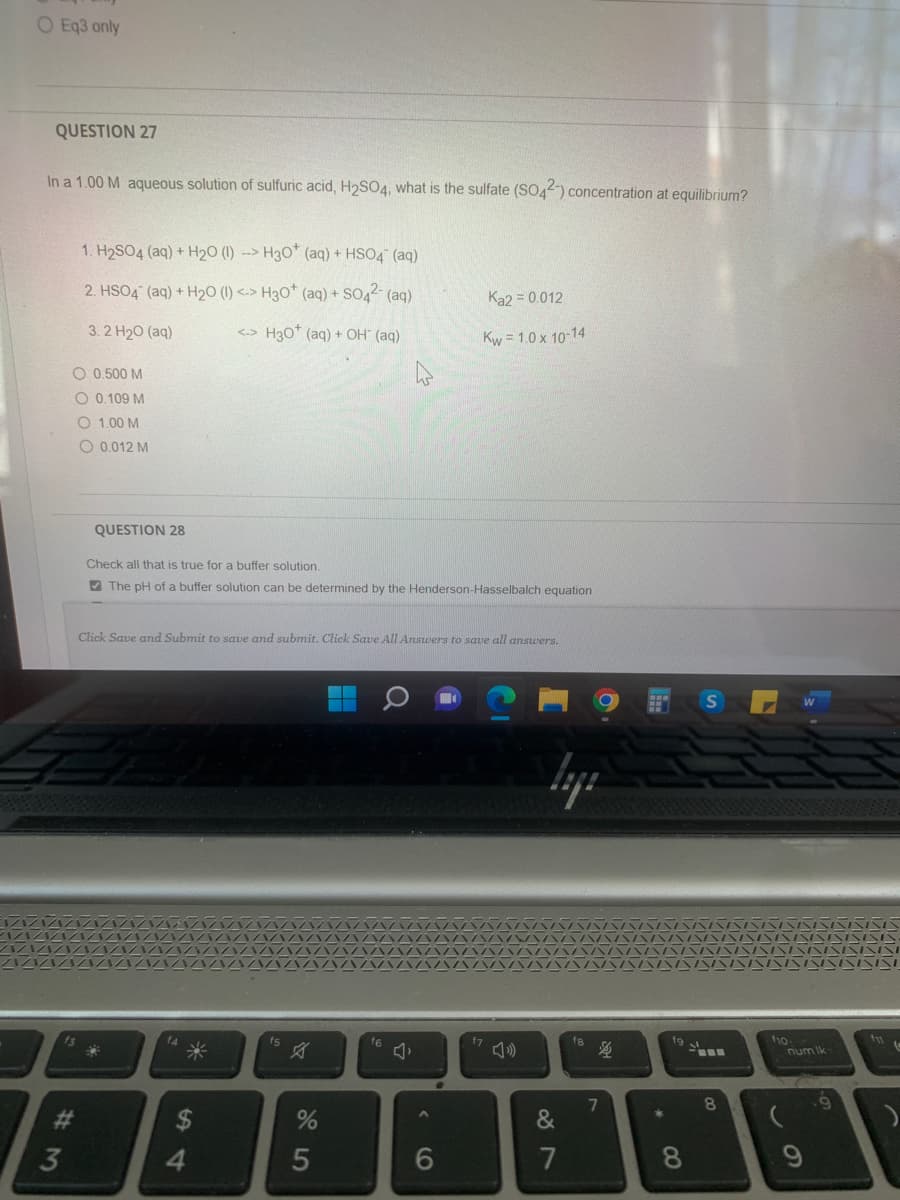
Transcribed Image Text:O Eq3 only
QUESTION 27
In a 1.00 M aqueous solution of sulfuric acid, H₂SO4, what is the sulfate (SO42-) concentration at equilibrium?
1. H₂SO4 (aq) + H₂O (1) --> H30+ (aq) + HSO4 (aq)
2. HSO4 (aq) + H₂O (1) <-> H3O+ (aq) + SO4²- (aq)
3. 2 H2O (aq)
<-> H30* (aq) + OH- (aq)
O 0.500 M
O 0.109 M
O 1.00 M
O 0,012 M
#3
QUESTION 28
Check all that is true for a buffer solution.
The pH of a buffer solution can be determined by the Henderson-Hasselbalch equation
54
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
4
fs
%
4
5
f6
Ka2 = 0.012
Kw=1.0 x 10-14
6
87
&
f8
4
7
fg
* 00
8
8
f10:
num Ik
9
.9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax