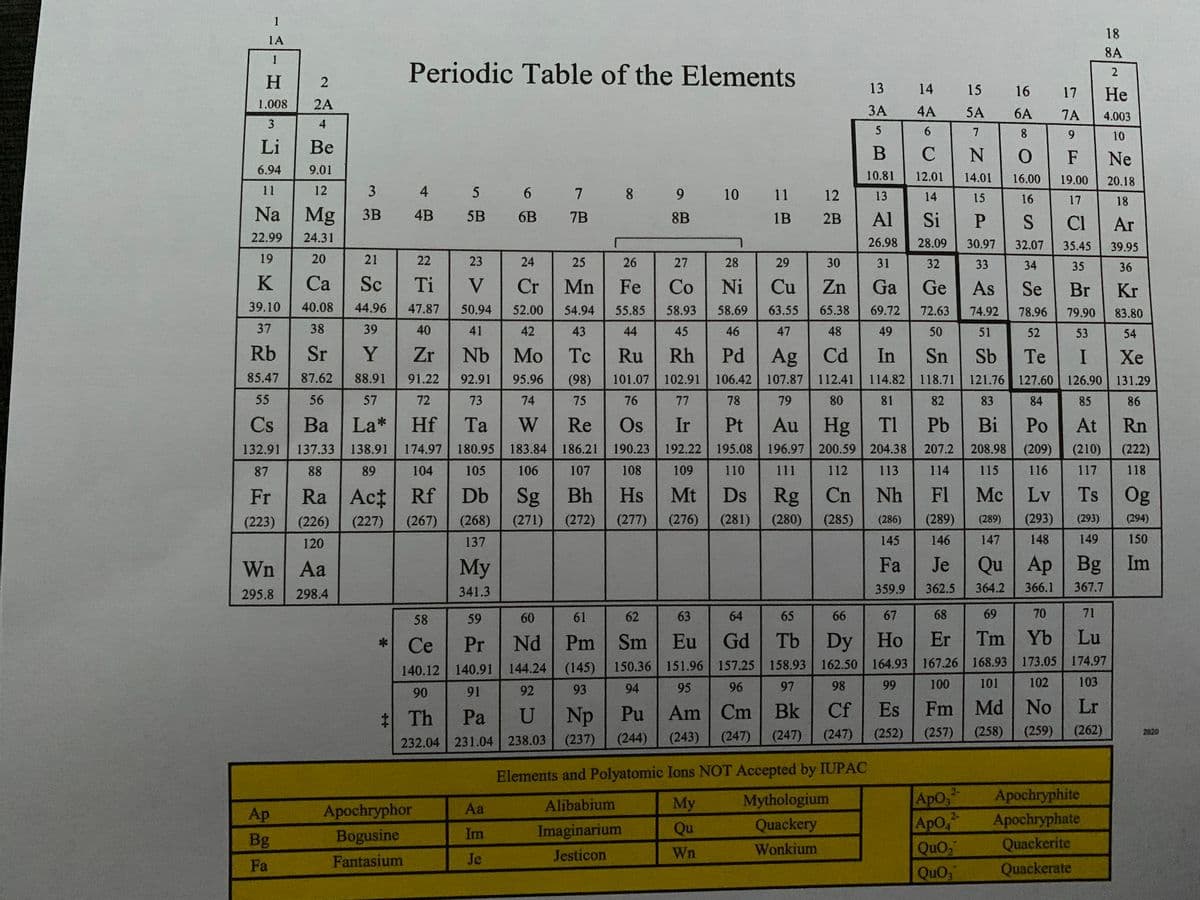
Question 11- Two isotopes of the element Fa occur naturally, with 282145 Fa At 23.4% (281.7 amu) and 275145 Fa at 76.6% (274.6 amu). Calculate the atomic mass for Fa using the weighted average mass method. (Please look at attached images for the two isotopes in this question, also look at attached images for a special periodic table you may need to solve this question. Please show all your work so I can understand going forward)
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question 11- Two
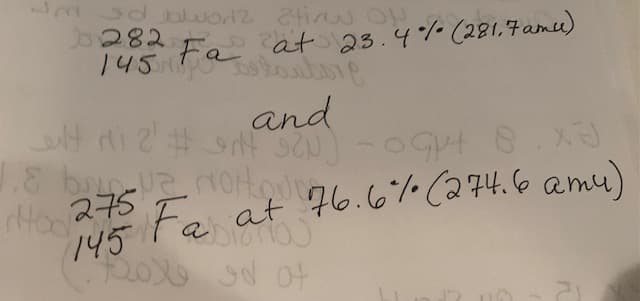
Trending now
This is a popular solution!
Step by step
Solved in 2 steps




