QUESTION 11 Which one is a wrong statement about the intermediate of nucleophilic acyl subsitution? The intermediate is a result of forming a new T bond. The step in mechanism after intermediate formation is reforming carbonyl group and loss of leaving group. O it is a tetrahedral intermediate. In the intermediate, O from carbonyl group is negatively charged. After nucleophilic attack step, the attacked carbon in the intermediate has sp hybridization. O O O
QUESTION 11 Which one is a wrong statement about the intermediate of nucleophilic acyl subsitution? The intermediate is a result of forming a new T bond. The step in mechanism after intermediate formation is reforming carbonyl group and loss of leaving group. O it is a tetrahedral intermediate. In the intermediate, O from carbonyl group is negatively charged. After nucleophilic attack step, the attacked carbon in the intermediate has sp hybridization. O O O
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.64P: 4-64 Aspirin is made by the reaction of salicylic acid with acetic anhydride. How many grams of...
Related questions
Question
Please answer both the parts of this question otherwise I'll downvote

Transcribed Image Text:QUESTION 11
Which one is a wrong statement about the intermediate of nucleophilic acyl subsitution?
The intermediate is a result of forming a new m bond.
The step in mechanism after intermediate formation is reforming carbonyl group and loss of leaving group.
O it is a tetrahedral intermediate.
In the intermediate, O from carbonyl group is negatively charged.
After nucleophilic attack step, the attacked carbon in the intermediate has spi hybridization.
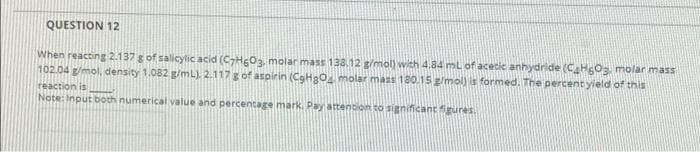
Transcribed Image Text:QUESTION 12
When reacting 2.137 g of salicylic acid (CHgOa molar mass 138.12 g/mol) with 4.84 ml of acecic anhydride (C HOs molar mass
102.04 g/mol, density 1.082 g/mL). 2.117g of aspirin (CgH2O molar mass 180.15 g/mol) is formed. The percent yield of this
reaction is
Note: Input both numerical value and percentage mark. Pay attention to significantgures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax