QUESTION 12 The highly toxic "antifreeze" has the formula C2H602. In your chemistry assignment you have been asked to observe what happens to 25 moles of antifreeze in a reaction. What is the total mass of the 25 moles of antifreeze if hydrogen, carbon, and oxygen have a molar mass of 1.008 gmol, 12.01 g/mol, and 16.00 g/mol, respectively? Keep answer to the tenth. Provide unit symbol. QUESTION 13 Masses of different types of bactorium vary. You have been informed in a health lab that 20 bacteria had a mass of 1.03 x 10g each and 11 bacteria had a mass of 9.9 x 10"g each. What was the total mass? Keep in scientific notation. Round mantissa to hundredth: Mantissa is Exponent for power of 10 is Unit symbol is Click Save and ubmit to soue and submit. Click Save All Ansuers to save all ansmuers Sen
QUESTION 12 The highly toxic "antifreeze" has the formula C2H602. In your chemistry assignment you have been asked to observe what happens to 25 moles of antifreeze in a reaction. What is the total mass of the 25 moles of antifreeze if hydrogen, carbon, and oxygen have a molar mass of 1.008 gmol, 12.01 g/mol, and 16.00 g/mol, respectively? Keep answer to the tenth. Provide unit symbol. QUESTION 13 Masses of different types of bactorium vary. You have been informed in a health lab that 20 bacteria had a mass of 1.03 x 10g each and 11 bacteria had a mass of 9.9 x 10"g each. What was the total mass? Keep in scientific notation. Round mantissa to hundredth: Mantissa is Exponent for power of 10 is Unit symbol is Click Save and ubmit to soue and submit. Click Save All Ansuers to save all ansmuers Sen
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 159IL: If Epsom salt, MgSO4 x H2O, is heated to 250 C, all the water of hydration is lost. On heating a...
Related questions
Question
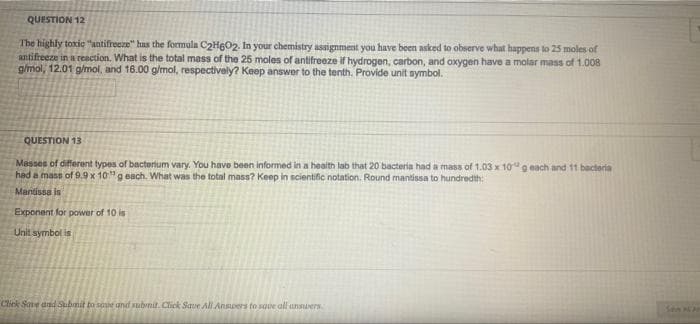
Transcribed Image Text:QUESTION 12
The highly toxic "antifreeze" has the formula C2H6O2. In your chemistry assignment you have been asked to observe what happens to 25 moles of
antifreeze in a reaction. What is the total mass of the 25 moles of antifreeze if hydrogen, carbon, and oxygen have a molar mass of 1.008
gmol, 12.01 g/mol, and 16.00 g/mol, respectively? Keep answer to the tenth. Provide unit symbol.
QUESTION 13
Masses of different types of bacterium vary. You have been informed in a health lab that 20 bacteria had a mass of 1.03 x 10g each and 11 bacteria
had a mass of 9.9 x 10"g each. What was the total mass? Keep in scientific notation. Round mantissa to hundredth:
Mantissa is
Exponent for power of 10 is
Unit symbol is
Click Save and Submit to saue and submit. Click Saue All Ansuers to save all ansners
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning