
Concept explainers
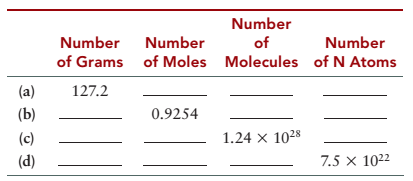
Complete the following table for TNT (trinitrotoluene), C7H5(NO2)3.
Interpretation:
The given table should be completed.
Concept introduction:
The number of moles of a substance is related to mass and molar mass as follows:
Here, m is mass and M is molar mass of the substance.
Also, according to Avogadro’s law in 1 mol of a substance there are
The density of a substance is related to mass and volume as follows:
Here, m is mass and V is volume.
Answer to Problem 13QAP
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Explanation of Solution
The given compound is TNT (trinitrotoluene) with molecular formula
The molar mass of carbon, hydrogen, nitrogen and oxygen is 12 g/mol, 1 g/mol, 14 g/mol and 16 g/mol respectively.
Putting the values,
Step (a)
The mass of TNT is 127.2 g. The number of moles can be calculated as follows:
Putting the values,
Since, according to Avogadro’s law in 1 mol of a substance there are
Thus, number of molecules in 0.56 mol of TNT will be:
Thus, number of molecules of TNT is
Now, the molecular formula of TNT is
Thus, number of N atoms will be:
Therefore, number of N atoms is
Step (b)
The number of moles of TNT is
Putting the values,
The number of molecules of TNT can be calculated as follows:
Now, in 1 mol there are 3 nitrogen atoms. Thus, the number of N atoms will be 3 times the number of molecules of TNT.
Step (c)
The number of molecules of TNT is
Thus, number of N atoms in
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Step (d)
The number of N atoms is
Since, the number of N atoms is 3 times the number of TNT molecule. Thus, number of molecules of TNT will be:
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Therefore, the complete table will be as follows:
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: Principles and Reactions
- Balance the following equations by filling in the blanks. (a) 92235U+01n54137_+201n+_ (b) 90232Th+612__01n+96240Cm (c) 24He+4296Mo43100_+_ (d) _+12H84210_+01narrow_forwardWhat are the live most abundant elements (by mass) in the earth’s crust, oceans, and atmosphere?arrow_forwardThe percentage of oxygen by weight in Al2(SO4)3 atomic weights: Al=27, S=32, O=16 is approximately: a. 19. b. 21. c. 56. d. 92.arrow_forward
- Polystyrene can be prepared by heating styrene with tribromobenzoyl peroxide in the absence of air. A sample prepared by this method has the empirical formula Br3C6cH3(C8H8)n, where the value of n can vary from sample to sample. If one sample has 0.105% Br, what is the value of n?arrow_forward2.75 Chlorine has only two isotopes, one with mass 35 and the other with mass 37. One is present at roughly 75% abundance, and the atomic weight of chlorine on a periodic table is 35.45. Which must be the correct mass spectrum for chlorine?arrow_forwardTwo basic laws of chemistry are the law of conservation of mass and the law of constant composition. Which of these laws (if any) do the following statements illustrate? (a) Lavoisier found that when mercury(ll) oxide, HgO, decomposes, the total mass of mercury (Hg) and oxygen formed equals the mass of mercury(ll) oxide decomposed. (b) Analysis of the calcium carbonate found in the marble mined in Carrara, Italy, and in the stalactites of the Carlsbad Caverns in New Mexico gives the same value for the percentage of calcium in calcium carbonate. (c) Hydrogen occurs as a mixture of two isotopes, one of which is twice as heavy as the other.arrow_forward
- The mass spectrum of bromine (Br2) consists of three peaks with the following characteristics: Mass (u) Relative Size 157.84 0.2534 159.84 0.5000 161.84 0.2466 How do you interpret these data?arrow_forwardPolyisobutylene is a synthetic elastomer, or rubber. The corresponding monomer is isobutylene, which has the molecular formula C4H8. What is the empirical formula of isobutylenearrow_forward9.21 x10^19 molecules of hydrogen gas,(H2) are equal to how many grams of hydrogen gas?arrow_forward
- The mass of 6 million molecules of a diatomic gas is 5.1 × 10-19 kg. What is the atomic mass of the element in this gas?arrow_forwardHow many grams of K2C2O4, potassium oxalate, would containe 182 grams of pure potassium?arrow_forwardGive the number of atoms of the specified element in aformula unit of each of the following compounds, and calculatethe molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C₆H₅COONH₄(b) Nitrogen in hydrazinium sulfate, N₂H₆SO₄(c) Oxygen in the mineral leadhillite, Pb₄SO₄(CO₃)₂(OH)₂arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





