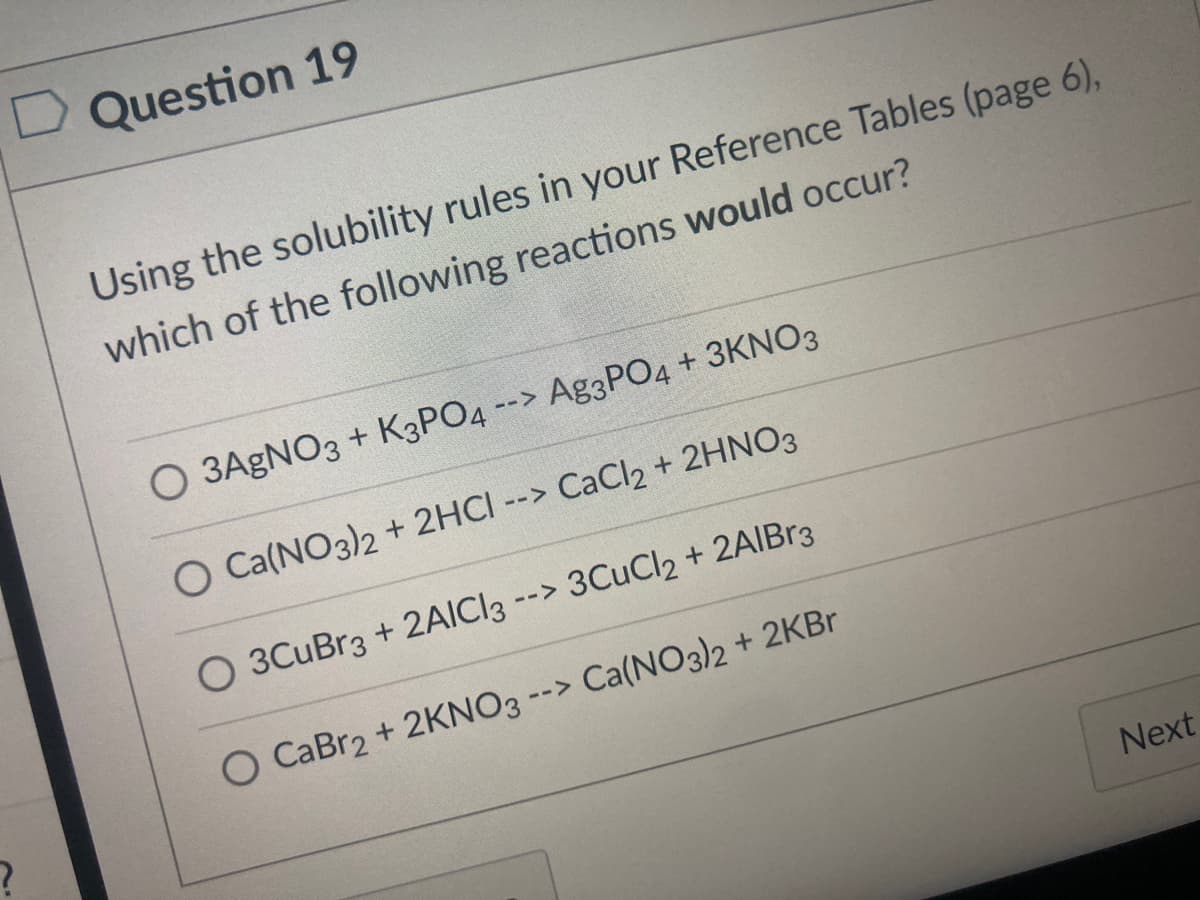
Question 19 Using the solubility rules in your Reference Tables (page 6), which of the following reactions would occur? O 3AGNO3 + K3PO4 --> Ag3PO4 + 3KNO3 O Ca(NO3)2 + 2HCI --> CaCl2 + 2HNO3 O 3CuBr3 + 2AICI3 --> 3CUCI2 + 2AIBR3 O CaBr2 + 2KNO3 --> Ca(NO3)2 + 2KB Next
Q: A permanganate solution is standardized by titrating it with a 0.1020 M hydrogen peroxide solution.…
A: a. Calculating the the concentration of the permanganate (MnO4-) solution:
Q: For Questions 20-24 consider the following scenario: A 0.8390 g of an unknown sample containing…
A:
Q: A person’s blood alcohol (C₂H₅OH) level can be determined by titrating a sample of blood plasma with…
A:
Q: 0.261 g of potassium hydrogen phthalate (KHP) was titrated with NaOH. When 16.8 mL of the NaOH had…
A: The molarity of a substance is defined as the number of moles of solute present per liter of…
Q: During a titration the following data were collected. A 50.0 mL portion of an HCl solution was…
A: HCl is strong acid and NaOH is strong base . In the titration reaction of HCl with NaOH , one mole…
Q: Which one of the following would form a precipitate when mixed with aqueous Ba(NO3)2? A)Na2SO4…
A:
Q: According to the solubility guidelines given in your lab manual, which of the following substances…
A: Given,
Q: When the following solutions are mixed together, what precipitate (if any) will form? a. FeSO4 +…
A:
Q: If 766 mL of 1.52 M sodium phosphate solution reacts with 109.3 mL of 2.63 M lead(II) nitrate…
A: Balanced chemical equation: 2Na3PO4(aq) + 3Pb(NO3)2(aq) ⟶ Pb3(PO4)2(s) + 6NaNO3(aq) Number of…
Q: A solution contains 0.0015 M Ag+ and 1.6 x 10-5 M Zn2+, and the ions need to be separated. For this…
A: Given data: A solution contain both 0.0015 M Ag+ and 1.6 x 10-5 M Zn2+, and the ions need to be…
Q: Milk of magnesia (magnesium hydroxide) reacts with stomach acid (hydrochloric acid). Calculate the…
A: Correct : 0.583 M Given neutralisation reaction; Mg(OH)2 + 2HCl ⟷⟷ MgCl2 + 2H2O Given volume of…
Q: [Review Topics] [References] Use the References to access important values if needed for this…
A: Mole is the amount of the substance that contains the same number of particles or atoms or…
Q: unlv.instructure.com 2. Write the formula of the substance produced when a copper (II) ion is…
A: a) the formula of hydroxide ion is OH- Since Copper (II) ion will be Cu2+ hence to balance the 2+…
Q: For Questions 20-24 consider the following scenario: A 0.8390 g of an unknown sample containing…
A: Given data,
Q: engine.html?ClassID=1746475745# Orie. 3.08 x 10-3 mol FeCl3 was identified through titration from…
A:
Q: please look at attached images for a special periodic table you may need to use to solve this…
A: Chemical reactions are those reactions which undergo any chemical change. Chemical reaction is…
Q: A 0.500-g sample of stannous fluoride gives a 0.590-g precipitate of stannous phosphate. Calculate…
A: Balance Chemical Reaction => 3 SnF2 (aq) + 2 Na3PO4 (aq) ---> Sn3(PO4)2 (s) + 6 NaF (aq)…
Q: Use the solubility generalizations on the information page to predict if one or more precipitates…
A:
Q: Which of these compounds are soluble in water? Use the solubility rules e and the periodic table e…
A:
Q: Using the solubility rules, determine whether the following compounds are soluble or insoluble.…
A: Solution : Solubility is very helpful to determine whether a compound is soluble or not. The…
Q: Which of the mixtures of compounds below will not produce a precipitate? MgBr2(aq) + AgClO4(aq)…
A: Precipitation reaction are those which form precipitates.
Q: A 6.586-g sample containing magnesium chloride and sodium chloride was dissolved in sufficient water…
A: Mass of sample = 6.586 g Mass of sample solution = 500 mL Mass of AgCl formed from 50 mL solution =…
Q: Use the solubility rules table to answer this question. Which compound is likely soluble in water…
A: If Ksp ( solubility product) is lower than ionic product, then precipitation occurs.
Q: A certain local brand of "healthy chicharon was found to contain 0.4132 g of NaCl after Fajan…
A: Given, Mass of NaCl = 0.4132 g Molar mass of NaCl = 58.44 g/mole Molar mass of Na+ = 22.99 g/mole…
Q: A 6.881-g sample containing magnesium chloride and sodium chloride was dissolved in sufficient water…
A: Given: mass of MgCl2·6H2O and NaCl sample = 6.881 g. Volume of solution made = 500 mL = 0.500 L…
Q: To determine the concentration of some hydrochloric acid, a diver titrated it with 0.200 mol/L…
A: Molarity: To express the concentration of a solution, molarity is used. It is defined as the number…
Q: Sodium-ion (Na+) and Calcium ion (Ca2+) produce nearly the same color in a flame test (yellow and…
A: Sodium-ion (Na+) and Calcium ion (Ca2+) produce nearly the same color in a flame test (yellow and…
Q: Complete each of the following as a net ionic equation, indicating whether a precipitate forms. If…
A: 1) Since PbCl2 is insoluble salt Hence the reaction will happen forming a ppt of PbCl2 And since…
Q: Samples containing oxalate ion can be titrated with solutions containing permanganate ion under…
A: From the given reaction we can see that, 2 moles of KMnO4 ( or MnO4-) will react with 5 moles of…
Q: [References) Use the References to access important values if needed for this question. In the…
A: Formation of Precipitate when given reagents are added to aqueous solution of Ba+2 and Cu+2 aqueous…
Q: 12.63 g sample of calcium ore was dissolved in HCI and gravimetrically analyzed, through the…
A: Theoritically :- 1 mole CaCO3 is giving 1 mole of Ca., n(Ca) = n(CaCO3) Moles of CaCO3 = mass of…
Q: A sample that weighed 0.8112 g is analyzed for phosphorus (MM= 30.9738 g/mol) content by…
A: The question is based on quantitative analysis. we have to quantitatively estimate the percentage…
Q: When excess Na,So, solution was added to 20.0 mL of an unknown solution containing Ca 0.0472 g of…
A: The question is based on the concept of Reaction Stoichiometry and solutions. We have to identify…
Q: Use the following equations to answer this question: A 12 +2e- 21 B SO4²- + H+ + e- H₂SO3 + H₂O CIO…
A: Chemical formula of bleach is NaClO. Reaction of bleach with Fe2+ ions is a redox reaction - a…
Q: Name Formula lonic/Covalent Soluble/Insoluble Electrolyte/Precipitate Ammonium Sulfide (NH4)2S…
A: The solution to the table is given below:
Q: Use the solubility rules from your textbook to answer the following questions regarding mixtures of…
A: (1) If a test tube mixture contain Ca2+ and Ag+ion containing salt. Now if we add sodium chloride…
Q: Question 12: On the basis of the general solubility rules, predict which of the following substances…
A: The solubility of an ionic compound in water depends on the nature of the ions present in the ionic…
Q: +2 When excess Na,so, solution was added to 20.0 mL of an unknown solution containing Ca ion, 0.0472…
A:
Q: Calculate the grams of analyte per gram of precipitate for the following conversions: Substance…
A: The grams of analyte per gram of precipitate is known as Gravimetric factor (G.F.) G.F. =a× Formula…
Q: Solution 1 Solutlon 2 OH O CHOO- O Na* O OH- O CHOOH Solution 3 Solution 4 OH* O cr O H* O cr
A: Solution 2) contains NaOH and Solution 3) contains HCl The reaction takes place is NaOH + HCl…
Q: Based on the solubility rules, which one of these compounds is insoluble in water? Select one: O a.…
A:
Q: A chemist performs a gravimetric analysis. The chemist combines 1.00 L of 2.00 M AGNO, (ag) with…
A: when aqueous solution of AgNO3 and NaCl are mixed , they react and form aqueous NaNO3 and solid…
Q: When excess lead(II) nitrate was added to 225 mL of a solution of potassium iodide, a solid…
A: given, Pb(NO3)2 (aq)+ 2KI (aq)-----> KNO3 (aq) + PbI2 (s) mass of PbI2 = 7.25 g volume = 225 ml…
Q: Milk of magnesia (magnesium hydroxide) reacts with stomach acid (hydrochloric acid). Calculate the…
A:
Q: Answer all the question below, 1. Phosphate ion (PO4) is determined by converting it to ammonium…
A:
Q: D Question 27 Which combination of aqueous solutions will form a precipitate when mixed O aqueous…
A: To predict the formatin of pecipitate, first we must know about the solubility Of compounds in…
Q: Which of these compounds are soluble in water? Use the solubility rules e and the periodic table @…
A:
Q: 6. A 6.881-g sample containing magnesium chloride and sodium chloride was dissolved in sufficient…
A:
Q: What mass of solid AgCl will precipitate from a solution containing 1.50g CaCl2 with AgNO3 in…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- 2. Write all mass balance (MB) and charge balances (CB) equations for the following: (1) 0.2 M K3PO4 + 0.3 M H3PO4 (2) 0.3 M NaAc + 0.1 M sucrose ( Note: Sucrose is C12H22O11, a non-electrolyte )1. A sample that weighed 0.8112 g is analyzed for phosphorus (MM= 30.9738 g/mol) content by precipitating the phosphorus as magnesium pyrophosphate, Mg2P2O7 (MM = 222.555 g/mol). If the precipitate weighs 0.5261 g, what is the percent of P in the sample?Required to answer. Single line text. 2. A 1.000 g sample containing sodium oxalate,Na2C2O4 (MM=126 g/mol) is titrated with 40.00 mL of 0.0200 M potassium permanganate, KMnO4 in acid solution. Calculate the percentage of sodium oxalate in the sample.Required to answer. Single line text. 5 moles C204 2 MNO4 +16HGravimetric analysis. Please show complete solution. Thank you Question: A 3.00- g sample of an alloy (containing only Pb and Sn) was dissolved in nitric acid (HNO3). Sulfuric acid was added to this solution, which precipitated 1.69 g of PbSO4. Assuming that all of the lead was precipitated, what is the percentageof Sn in the sample?
- CHEMISTRY (Please write the complete solution legibly. No long explanation needed. Answer in 2 decimal places. Box the final answer.) Solve the following: a. Calculate the molarity of hydrochloric acid, HCl, in a solution if 25.00 mL of that solution required35.74 mL of 0.1522 M KOH for complete neutralization in a titration. (Answer: 0.2176 M) b. When aqueous solutions of Ba(NO3)2 and K2SO4 are mixed, a precipitate of BaSO4 results. Writethe molecular, ionic, and net ionic equation for this double displacement reaction. c. 8.50 g of copper (II) sulfate pentahydrate, CuSO4∙5H2O, is dissolved in 250 mL of solution. Whatis the molarity of this solution?Need help with question 7 but 4-6 are here for context, thank you. Question 4 A standardized iron (II) solution that is 0.224 M in Fe2+ was used to determine the concentration of permanganate in solution. If the titration required 31.2 mL of Fe2+, how many moles of permanganate ion are present in solution? The pertinent balanced chemical equation is: MnO4- (aq) + 5 Fe2+ (aq) + 8 H+ (aq) --> Mn2+ (aq) + 5 Fe3+ (aq) + 4 H2O (l). Question 5 A standardized iron (II) solution that is 0.224 M in Fe2+ was used to determine the concentration of permanganate in solution. If the titration required 31.2 mL of Fe2+, how many moles of permanganate ion are present in solution? The pertinent balanced chemical equation is: MnO4- (aq) + 5 Fe2+ (aq) + 8 H+ (aq) --> Mn2+ (aq) + 5 Fe3+ (aq) + 4 H2O (l). Question 6 An ore containing solid titanium was processed so that it would yield titanium (IV) permanganate. A titration with an iron (II) standard was performed that indicated that…Gravimetric analysis. Please show complete solution. Thank you. Question: In the analysis of 0.7011 g of an impure chloride containing sample, 0.9805 g of AgCl were precipitated. What is the percentage by mass chloride in the sample?
- Write the balanced neutralization reaction that occurs between H2SO4H2SO4 and KOHKOH in aqueous solution. Phases are optional. neutralization reaction: Suppose 0.250 L0.250 L of 0.500 M H2SO40.500 M H2SO4 is mixed with 0.200 L0.200 L of 0.200 M KOH0.200 M KOH. What concentration of sulfuric acid remains after neutralization?What is the Molarity of KOH if a (2.3400x10^1) mL sample requires (4.27x10^1) mL of (3.6000x10^-1) M HNO3 in a titration experiment? Hint: Be sure to first write the balanced reaction. Enter your answer in scientific notation with 3 sig figs. Do not include any units in your answer. Do not round any intermediate calculations.Hi what is the net ionic equation when Barium is mixed with SCN^-? and net ionic equation when Barium is mixed with NO3? and when Barium is combined with CO3^-2? and when Barium is combined with SO4^-2? and when Barium is combined with Cl^-? and when Barium is combined with OH^-? and when Barium is combined with NO3^-? all solutions are kept in water/or diluted with water. thanks!
- How many grams of sample which contains 18.00% Na2O (61.98) should be taken for analysis in order to obtain a precipitate of Na3AsO3 (191.89) which weighs 0.3000 g?Write the balanced neutralization reaction that occurs between H2SO4H2SO4 and KOHKOH in aqueous solution. Phases are optional. neutralization reaction: Suppose 0.950 L0.950 L of 0.440 M H2SO40.440 M H2SO4 is mixed with 0.900 L0.900 L of 0.230 M KOH0.230 M KOH. What concentration of sulfuric acid remains after neutralization? concentration: M H2SO4How many mL of a 0.218 M Na3PO4 solution are required to precipitate all the calcium ions in a 395 mL of a 0.254 M CaCl2 solution. Go to 3 sig figs and do not use scientific notation

