Question 19 Which of the following does NOT describe an exothermic chemical reaction? O The temperature of the surroundings increases. O Heat is released by the reaction O The potential energy of the reactants is higher than the potential energy of the products O The enthalpy change (AH) is a positive value Question 20 Consider the following thermochemical equation for the combustion of propane C3Hg()+30₂(g) - 3C0₂(g) + 4H₂O(g) AH = -2220 kJ Determine the enthalpy change when 0.350 mol C₂H, reacts O-400 kJ O-17.6 kJ DEFM20 -34,300 kJ O-777 kJ O-0.634 kJ
Question 19 Which of the following does NOT describe an exothermic chemical reaction? O The temperature of the surroundings increases. O Heat is released by the reaction O The potential energy of the reactants is higher than the potential energy of the products O The enthalpy change (AH) is a positive value Question 20 Consider the following thermochemical equation for the combustion of propane C3Hg()+30₂(g) - 3C0₂(g) + 4H₂O(g) AH = -2220 kJ Determine the enthalpy change when 0.350 mol C₂H, reacts O-400 kJ O-17.6 kJ DEFM20 -34,300 kJ O-777 kJ O-0.634 kJ
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section1.8: Energy: Some Basic Principles
Problem 2RC
Related questions
Question
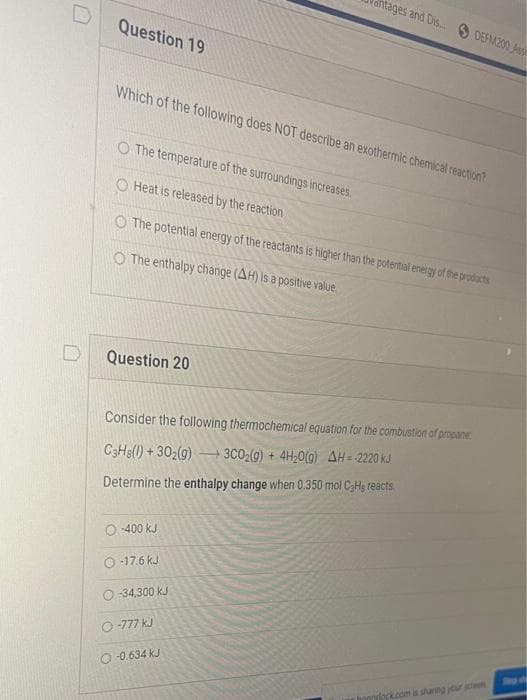
Transcribed Image Text:Question 19
Which of the following does NOT describe an exothermic chemical reaction?
O The temperature of the surroundings increases.
O Heat is released by the reaction
The potential energy of the reactants is higher than the potential energy of the products
O The enthalpy change (AH) is a positive value
Question 20
itages and Dis... DEFM200 Ass
Consider the following thermochemical equation for the combustion of propane
C3H8(1)+302(g) → 3C0₂(g) + 4H₂O(g) AH = 2220 kJ
Determine the enthalpy change when 0.350 mol CaHg reacts
O-400 kJ
O-17.6 kJ
O-34,300 kJ
O-777 kJ
-0.634 KJ
honolock.com is sharing your schem
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning