Question 2 The concentration of a diluted "wine" sample was found to be 0.32 %(v/v) ethanol. Assuming the dilution was done as described in Part B of the procedure for this lab, what was the original concentration of ethanol in the "wine"? Enter your answer with 2 significant figures.
Question 2 The concentration of a diluted "wine" sample was found to be 0.32 %(v/v) ethanol. Assuming the dilution was done as described in Part B of the procedure for this lab, what was the original concentration of ethanol in the "wine"? Enter your answer with 2 significant figures.
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
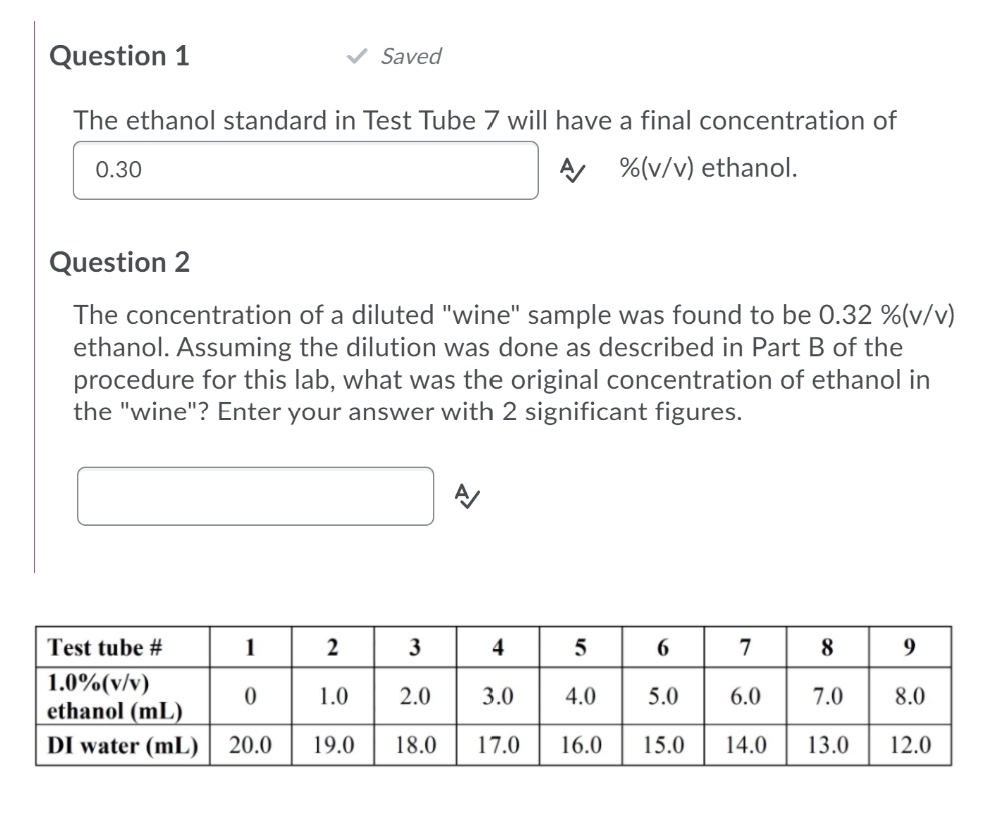
Transcribed Image Text:Question 1
Saved
The ethanol standard in Test Tube 7 wil| have a final concentration of
0.30
A %(v/v) ethanol.
Question 2
The concentration of a diluted "wine" sample was found to be 0.32 %(v/v)
ethanol. Assuming the dilution was done as described in Part B of the
procedure for this lab, what was the original concentration of ethanol in
the "wine"? Enter your answer with 2 significant figures.
Test tube #
1
4
8
9.
1.0%(v/v)
ethanol (mL)
DI water (mL) | 20.0
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.0
19.0
18.0
17.0
16.0
15.0
14.0
13.0
12.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

