Question 20 of 30 A calibrated burette will be used to deliver an intravenous medication dosage order of 200 mg contained in 5 mL. This volume is to be diluted in 40 mL and infused over 30 minutes. A 15-ml flush is to follow the infusion. At what rate in milliliters per hour will the infusion need to run to deliver the ordered dose? Record your answer as a whole number. mL/hr
Question 20 of 30 A calibrated burette will be used to deliver an intravenous medication dosage order of 200 mg contained in 5 mL. This volume is to be diluted in 40 mL and infused over 30 minutes. A 15-ml flush is to follow the infusion. At what rate in milliliters per hour will the infusion need to run to deliver the ordered dose? Record your answer as a whole number. mL/hr
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 113AE: Some of the physical properties of H2O and D2O are as follows: Property H2O D2O Density at 20C...
Related questions
Question
Transcribed Image Text:m-n- x herthHa
Eavier Aotive Qung ox
Sheerpwth Quir 7 PerentelX
Sherpam Maement Sy
agng eisevier.com//auirtansignmentid3455638courselda154811Atoken-eyJhbociOUIUUMU9.eydzdwiouprzht.mvdaGlua3NpdovitmvbscDAlinidXNci
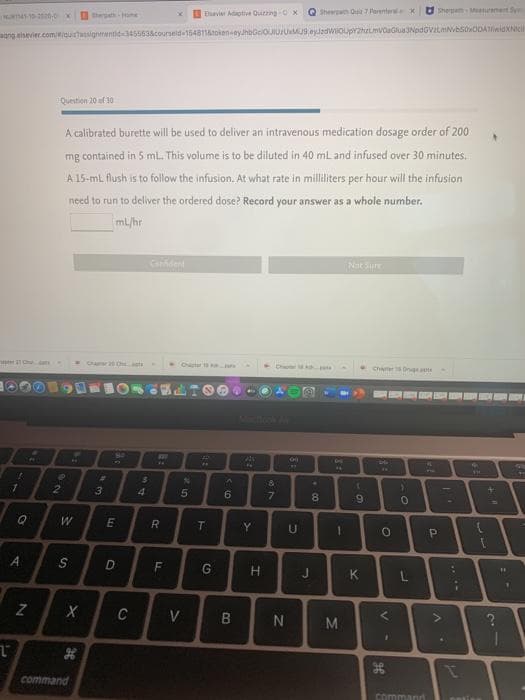
Question 20 of 30
A calibrated burette will be used to deliver an intravenous medication dosage order of 200
mg contained in 5 mL. This volume is to be diluted in 40 mL and infused over 30 minutes.
A 15-ml flush is to follow the infusion. At what rate in milliliters per hour will the infusion
need to run to deliver the ordered dose? Record your answer as a whole number.
mL/hr
Candent
Nat Sure
m C
Cha O ts
Ch
Cha On
3
6
7.
8.
R.
T.
Y.
U
A
K
C
M
command
comman
P.
CO
38
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning