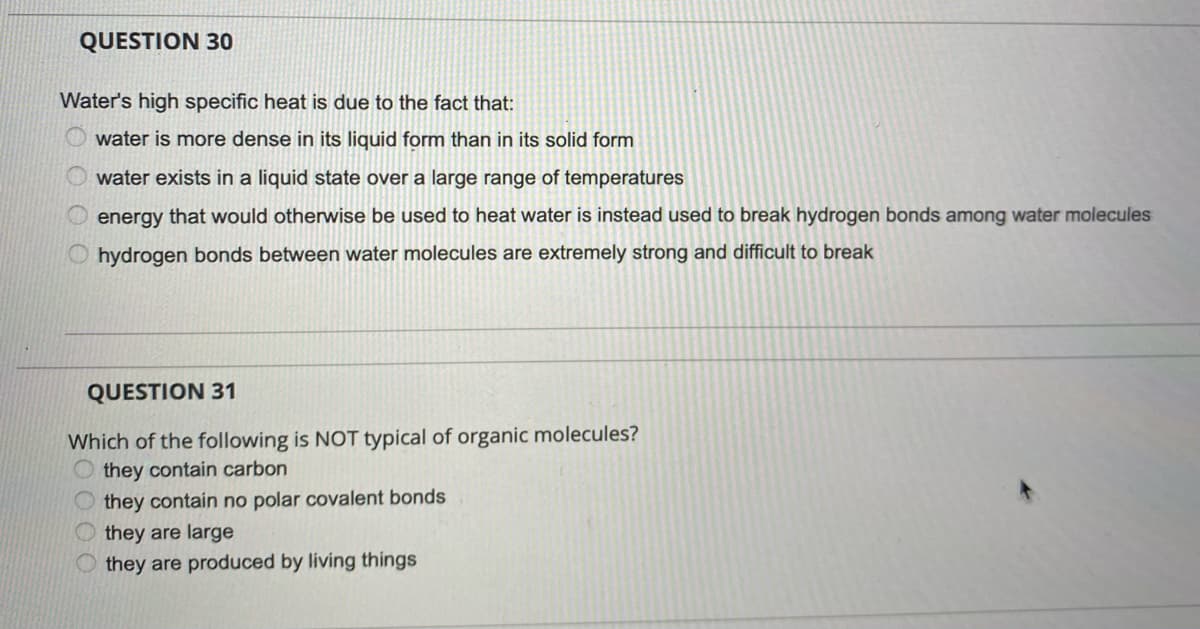
QUESTION 30 Water's high specific heat is due to the fact that: water is more dense in its liquid form than in its solid form water exists in a liquid state over a large range of temperatures energy that would otherwise be used to heat water is instead used to break hydrogen bonds among water molecules O hydrogen bonds between water molecules are extremely strong and difficult to break QUESTION 31 Which of the following is NOT typical of organic molecules? they contain carbon they contain no polar covalent bonds O they are large they are produced by living things
Nucleotides
It is an organic molecule made up of three basic components- a nitrogenous base, phosphate,and pentose sugar. The nucleotides are important for metabolic reactions andthe formation of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Nucleic Acids
Nucleic acids are essential biomolecules present in prokaryotic and eukaryotic cells and viruses. They carry the genetic information for the synthesis of proteins and cellular replication. The nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The structure of all proteins and ultimately every biomolecule and cellular component is a product of information encoded in the sequence of nucleic acids. Parts of a DNA molecule containing the information needed to synthesize a protein or an RNA are genes. Nucleic acids can store and transmit genetic information from one generation to the next, fundamental to any life form.
Step by step
Solved in 2 steps









