QUESTION 44 The following processes occurs in a reversible thermodynamic cycle: 1-2: Reversible polytropic compression at pressure 0.5 bar at volume 0.08 m to a pressure 11 bar and specific volume 0.4 m/kg. The index of compression may be taken as n. 2-3: Reversibly expansion with expansion index of 2 to pressure 2.9 bar. 3-1: Reversible cooling at constant volume to the initial state. Calculate the work for the expansion in the process to 2 decimal places. Not in kilo or mega of unit.
QUESTION 44 The following processes occurs in a reversible thermodynamic cycle: 1-2: Reversible polytropic compression at pressure 0.5 bar at volume 0.08 m to a pressure 11 bar and specific volume 0.4 m/kg. The index of compression may be taken as n. 2-3: Reversibly expansion with expansion index of 2 to pressure 2.9 bar. 3-1: Reversible cooling at constant volume to the initial state. Calculate the work for the expansion in the process to 2 decimal places. Not in kilo or mega of unit.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Transcribed Image Text:estion Completion StatuS:
(a)
(b)
(c)
(d)
O a. (d) O b.(a) Oc(c) Od.(b)
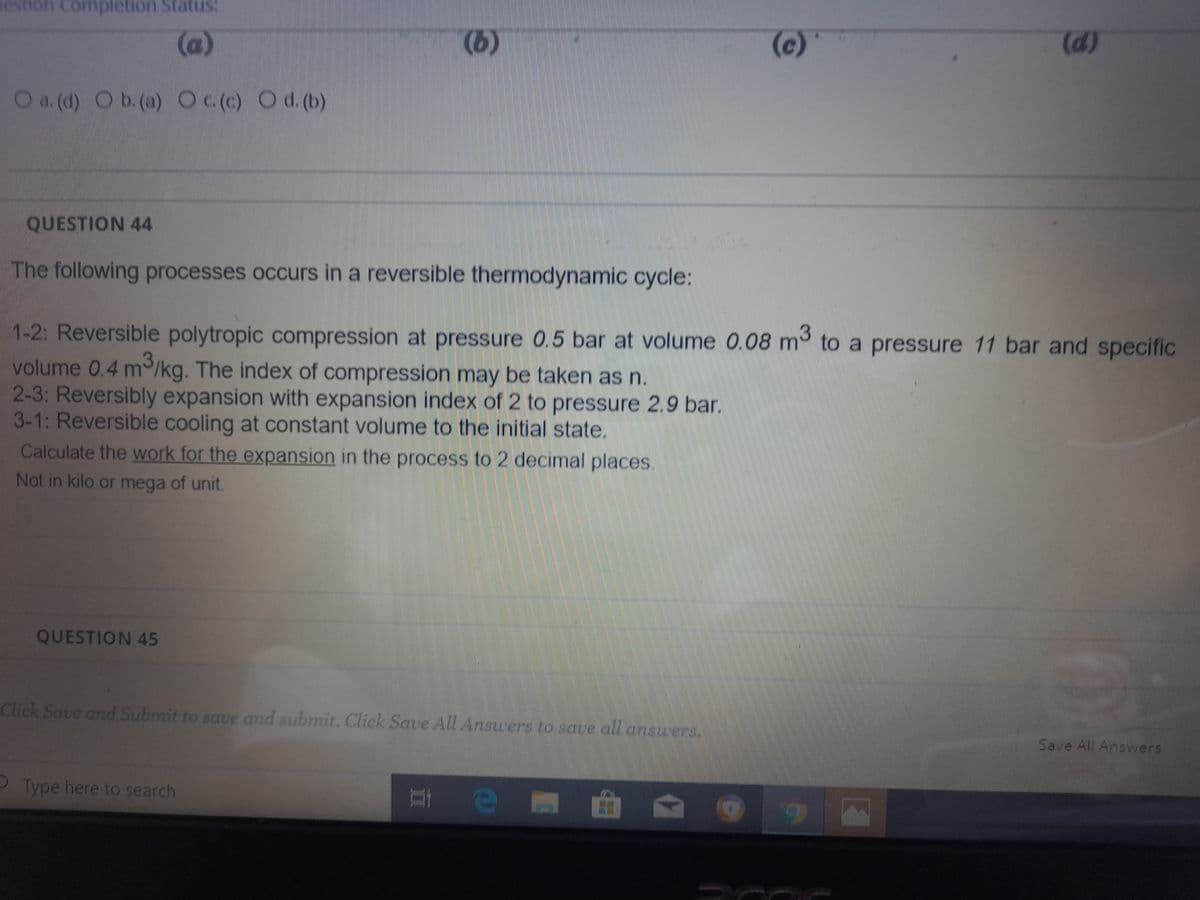
QUESTION 44
The following processes occurs in a reversible thermodynamic cycle:
1-2: Reversible polytropic compression at pressure 0.5 bar at volume 0.08 m to a pressure 11 bar and specific
volume 0.4 m/kg. The index of compression may be taken as n.
2-3: Reversibly expansion with expansion index of 2 to pressure 2.9 bar.
3-1: Reversible cooling at constant volume to the initial state.
Calculate the work for the expansion in the process to 2 decimal places.
Not in kilo or mega of unit.
QUESTION 45
Click Save and Submit to save and submit. Click Save All Answers to save all anszers.
Save All Answers
P Type here to search
曲
Transcribed Image Text:Question Completion Status:
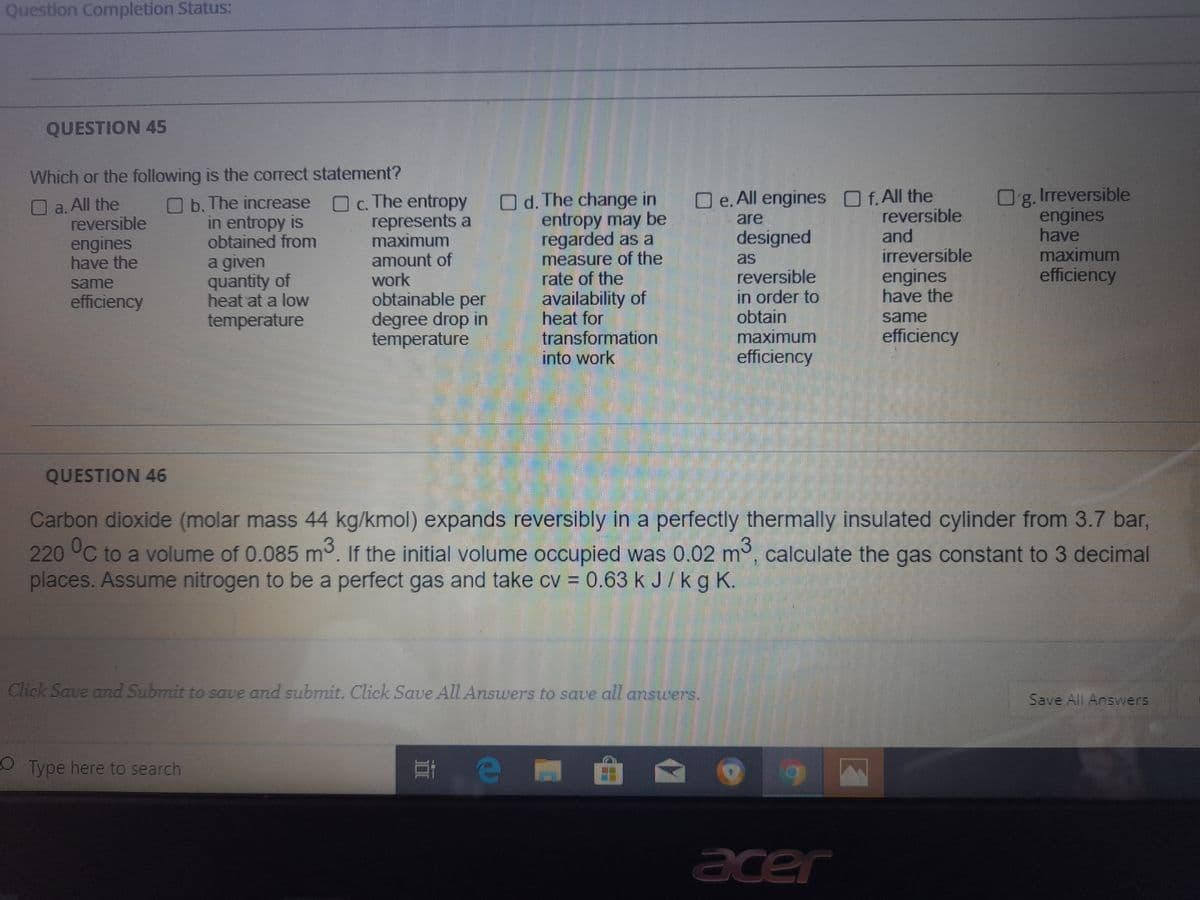
QUESTION 45
Which or the following is the correct statement?
Irreversible
Og.
engines
have
maximum
efficiency
O e. All engines f.All the
O c. The entropy
represents a
maximum
amount of
work
obtainable per
degree drop in
temperature
O d. The change in
entropy may be
regarded as a
measure of the
rate of the
availability of
heat for
transformation
into work
a.All the
reversible
O b. The increase
in entropy is
obtained from
reversible
and
irreversible
are
designed
engines
have the
as
a given
quantity of
heat at a low
engines
have the
reversible
in order to
obtain
maximum
efficiency
same
efficiency
same
temperature
efficiency
QUESTION 46
Carbon dioxide (molar mass 44 kg/kmol) expands reversibly in a perfectly thermally insulated cylinder from 3.7 bar,
220 °C to a volume of 0.085 m3. If the initial volume occupied was 0.02 m, calculate the gas constant to 3 decimal
places. Assume nitrogen to be a perfect gas and take cv = 0.63 k J/kg K.
Click Save and Submit to save and submit. Click Save All Answers to save all ansuers.
Save All Answers
O Type here to search
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY