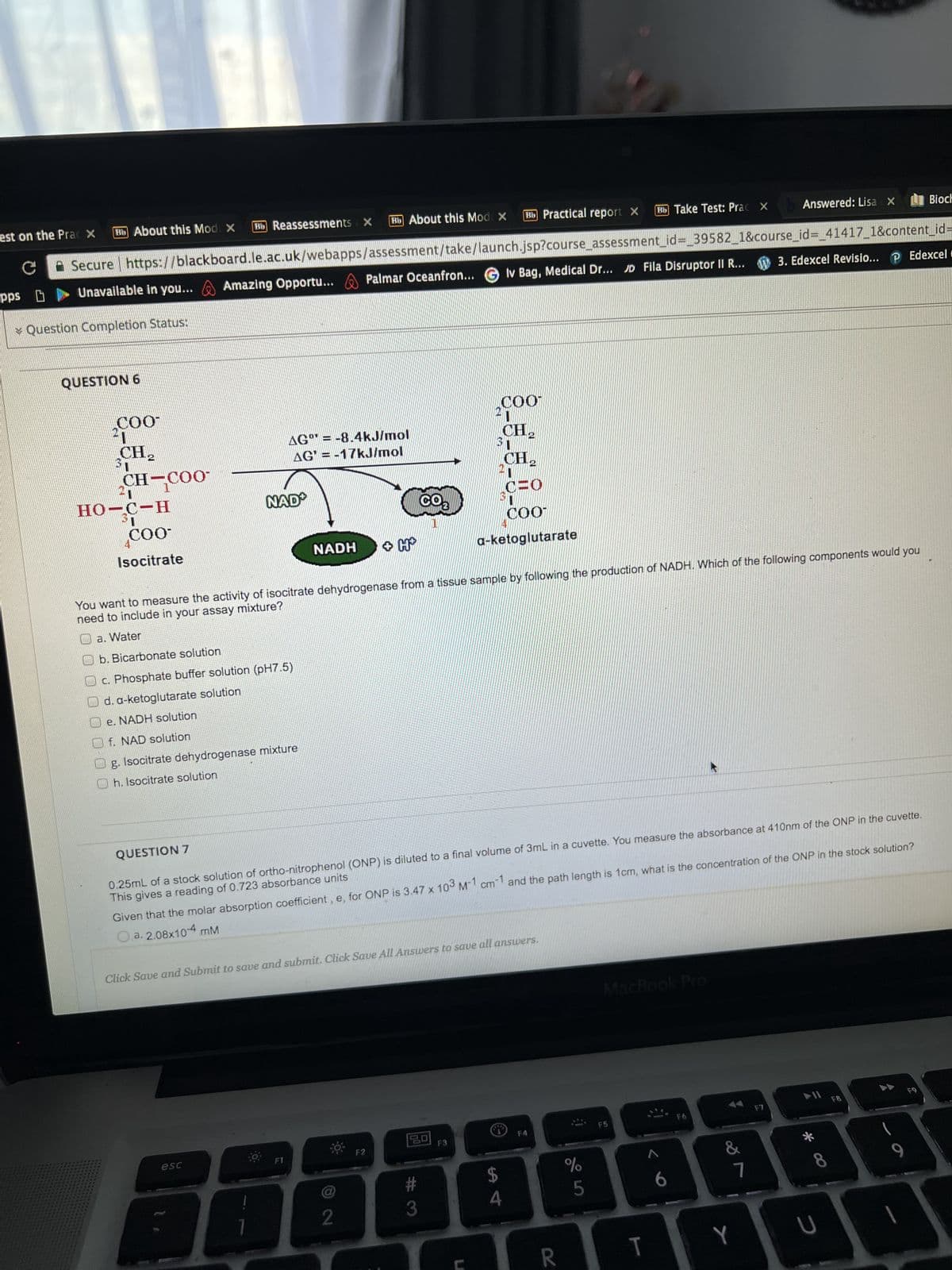
QUESTION 6 COO 21 CH₂ 31 CH-COO 21 HO-C-H 31 COO- Isocitrate AG= -8.4kJ/mol AG'= -17kJ/mol NAD NADH Oe. NADH solution f. NAD solution g. Isocitrate dehydrogenase mixture Oh. Isocitrate solution +H CO₂ COO 1 CH₂ 31 CH, CHO COO a-ketoglutarate You want to measure the activity of isocitrate dehydrogenase from a tissue sample by following the production of NADH. Which of the following components would you need to include in your assay mixture? a. Water Ob. Bicarbonate solution Oc. Phosphate buffer solution (pH7.5) d. a-ketoglutarate solution
QUESTION 6 COO 21 CH₂ 31 CH-COO 21 HO-C-H 31 COO- Isocitrate AG= -8.4kJ/mol AG'= -17kJ/mol NAD NADH Oe. NADH solution f. NAD solution g. Isocitrate dehydrogenase mixture Oh. Isocitrate solution +H CO₂ COO 1 CH₂ 31 CH, CHO COO a-ketoglutarate You want to measure the activity of isocitrate dehydrogenase from a tissue sample by following the production of NADH. Which of the following components would you need to include in your assay mixture? a. Water Ob. Bicarbonate solution Oc. Phosphate buffer solution (pH7.5) d. a-ketoglutarate solution
Chapter19: Calculating Iv Infusion And Completion Times
Section: Chapter Questions
Problem 2.6P
Related questions
Question
Transcribed Image Text:est on the Prac X
pps
Bb Reassessments X
Bb About this Modi X
* Question Completion Status:
QUESTION 6
Secure https://blackboard.le.ac.uk/webapps/assessment/take/launch.jsp?course_assessment_id=_39582_1&course_id=_41417_1&content_id=
Iv Bag, Medical
Palmar Oceanfron... Glv Bag, Medical Dr...
Amazing Opportu...
Unavailable in you...
COO
CH₂
CH-COO-
21
HO-C-H
S
COO-
Isocitrate
4
OL
AG = -8.4kJ/mol
AG' = -17kJ/mol
NAD
Bb About this Modi X Bb Practical report X
esc
NADH
2
+ H+
You want to measure the activity of isocitrate dehydrogenase from a tissue sample by following the production of NADH. Which of the following components would you
need to include in your assay mixture?
a. Water
b. Bicarbonate solution
c. Phosphate buffer solution (pH7.5)
d. a-ketoglutarate solution
e. NADH solution
f. NAD solution
g. Isocitrate dehydrogenase mixture
Oh. Isocitrate solution
F2
CO
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
QUESTION 7
0.25mL of a stock solution of ortho-nitrophenol (ONP) is diluted to a final volume of 3mL in a cuvette. You measure the absorbance at 410nm of the ONP in the cuvette.
This gives a reading of 0.723 absorbance units
Given that the molar absorption coefficient, e, for ONP is 3.47 x 103 M-1 cm-1 and the path length is 1cm, what is the concentration of the ONP in the stock solution?
Oa. 2.08x104 mM
20
#
2C007
CH₂
3
CH₂
C=0
000
a-ketoglutarate
3
G
F3
$
R
Bb Take Test: Prac X
%
5
Dr... JD Fila Disruptor II R... 3. Edexcel Revisio... p Edexcel
MacBook Pro
F5
T
Answered: Lisa X
F6
57
Y
* CO
8
U
Bioch
F8
a
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you


Case Studies In Health Information Management
Biology
ISBN:
9781337676908
Author:
SCHNERING
Publisher:
Cengage

Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage


Case Studies In Health Information Management
Biology
ISBN:
9781337676908
Author:
SCHNERING
Publisher:
Cengage

Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage