Question 7 of 20 The ions formed when Sr(C,H,O,), dissociates in water are A) Sr2", C*, H*, and O2 B) Sr+ and C,HO, C) Sr²* and C̟H,0; D) Sr2* and C,HO,* E) No ions form as Sr(C,H,O,), does not dissociate.
Question 7 of 20 The ions formed when Sr(C,H,O,), dissociates in water are A) Sr2", C*, H*, and O2 B) Sr+ and C,HO, C) Sr²* and C̟H,0; D) Sr2* and C,HO,* E) No ions form as Sr(C,H,O,), does not dissociate.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 8QAP: ow do chemists know that the ions behave independently of one another when an ionic solid is...
Related questions
Question
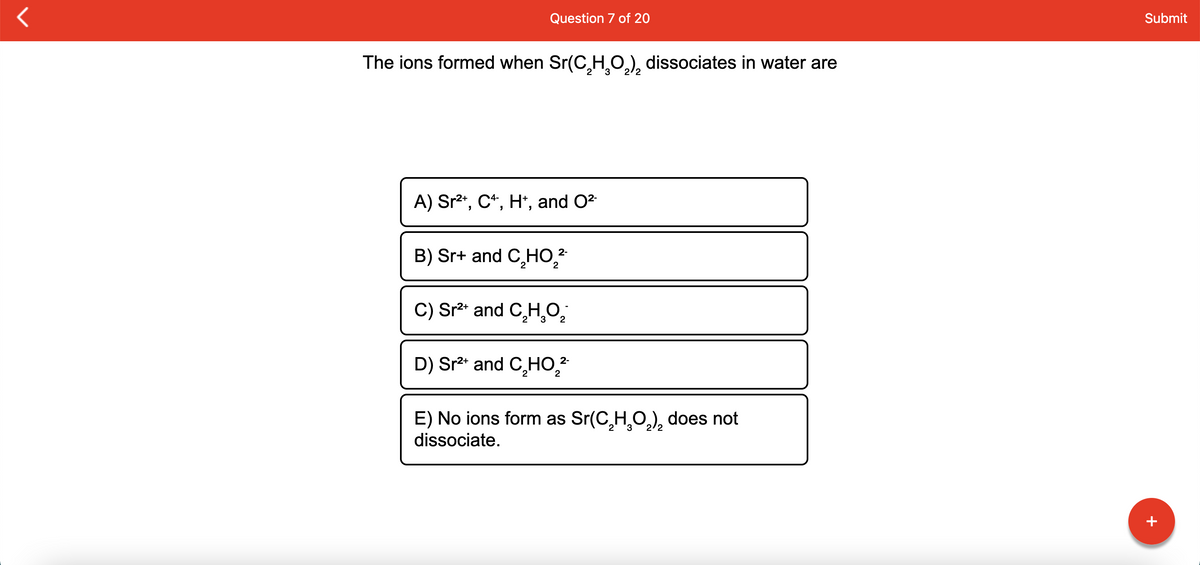
Transcribed Image Text:Question 7 of 20
Submit
The ions formed when Sr(C,H̟O.), dissociates in water are
2/2
A) Sr2*, C*, H*, and O2
B) Sr+ and C,HO,?
2-
C) Sr²* and C̟H,0;
D) Sr²* and C̟HO,?
2-
E) No ions form as Sr(C,H,0,), does not
dissociate.
2/2
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning