Question 8 A balanced equation showing hydrogen gas reacting with oxygen gas to form water could be interpreted several different ways. Which statement is an incorrect way to interpret the balanced equation? Your answer: O A) two moles of hydrogen gas react with one mole of oxygen gas to produce two moles of water vapor O B) two grams of hydrogen gas react with one gram of oxygen gas to produce two grams of water vapor C) two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water vapor D) two liters of hydrogen gas react with one liter of oxygen gas to produce two liters of water vapor Clear answer
Question 8 A balanced equation showing hydrogen gas reacting with oxygen gas to form water could be interpreted several different ways. Which statement is an incorrect way to interpret the balanced equation? Your answer: O A) two moles of hydrogen gas react with one mole of oxygen gas to produce two moles of water vapor O B) two grams of hydrogen gas react with one gram of oxygen gas to produce two grams of water vapor C) two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water vapor D) two liters of hydrogen gas react with one liter of oxygen gas to produce two liters of water vapor Clear answer
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 152CP
Related questions
Question
Help with both
Transcribed Image Text:Overview
Plans
Resources
Follow-up and reports
360° reports
More
1 2 3 4 5
6 7
8.
9.
10
11-20 21 -25
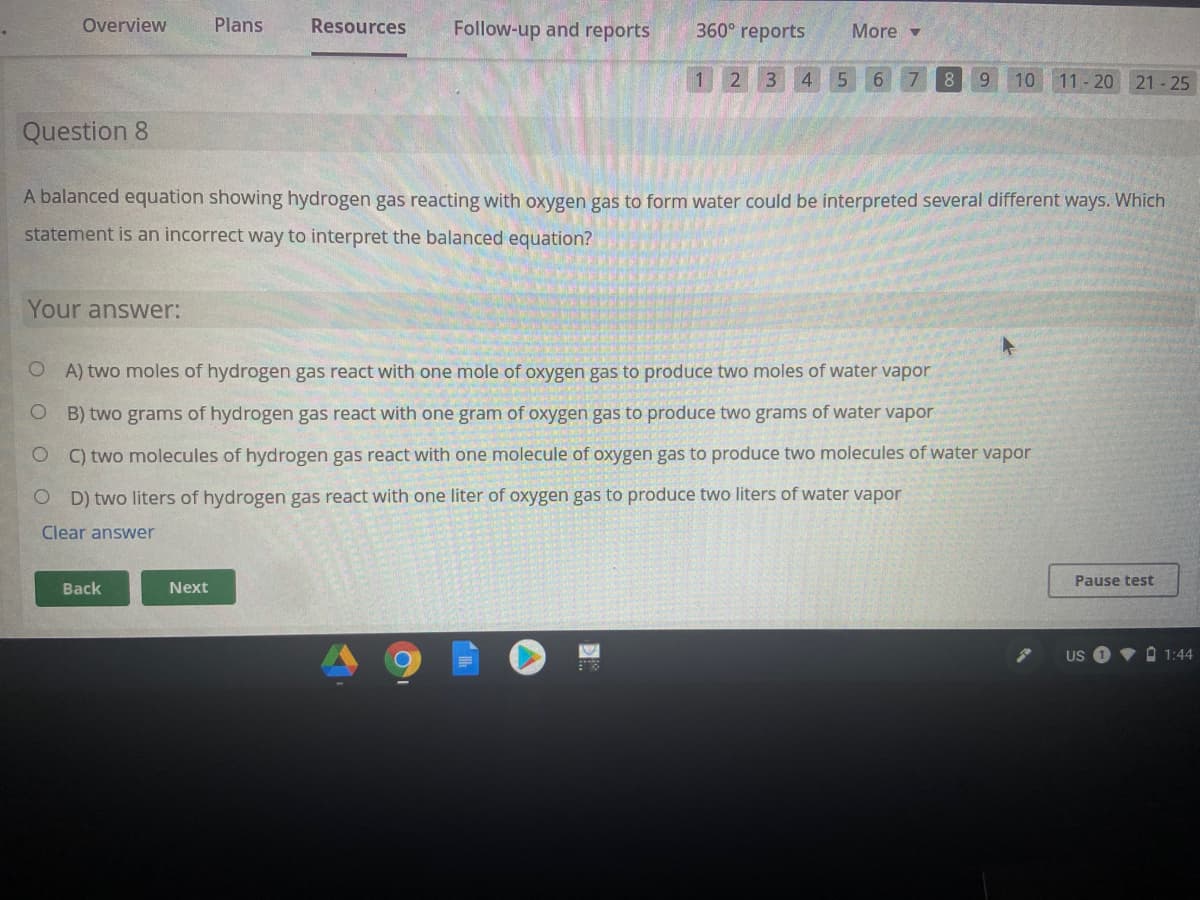
Question 8
A balanced equation showing hydrogen gas reacting with oxygen gas to form water could be interpreted several different ways. Which
statement is an incorrect way to interpret the balanced equation?
Your answer:
O A) two moles of hydrogen gas react with one mole of oxygen gas to produce two moles of water vapor
B) two grams of hydrogen gas react with one gram of oxygen gas to produce two grams of water vapor
C) two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water vapor
D) two liters of hydrogen gas react with one liter of oxygen gas to produce two liters of water vapor
Clear answer
Pause test
Back
Next
US
V O 1:44
Transcribed Image Text:ne Chem ...
Overview
Plans
Resources
Follow-u
口
Gases Quiz
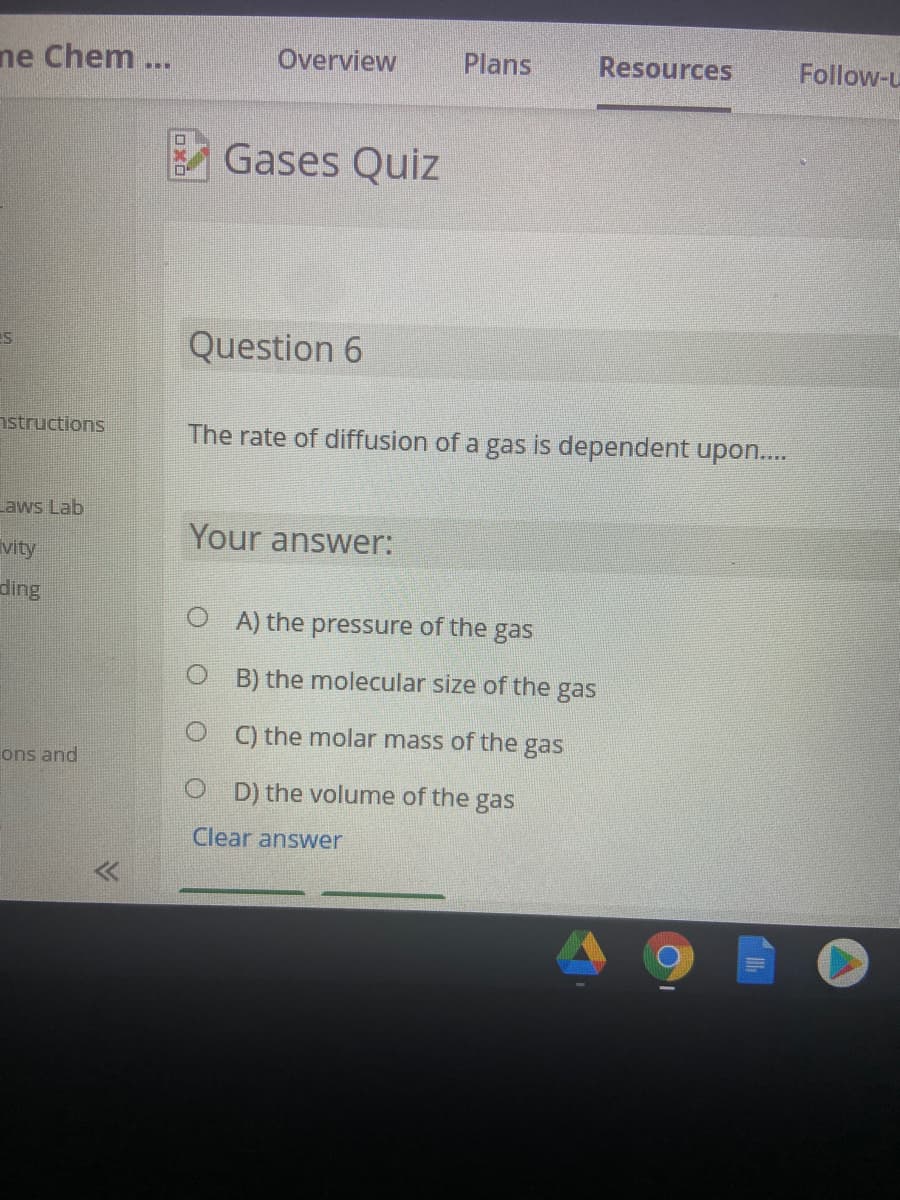
Question 6
istructions
The rate of diffusion of a gas is dependent upon..
Laws Lab
Your answer:
vity
ding
O A) the pressure of the gas
O B) the molecular size of the gas
O ) the molar mass of the gas
ons and
O D) the volume of the gas
Clear answer
Expert Solution
Step 1
Hello. Since you have posted multiple questions, we will solve only first question for you. If you want the remaining questions to be solved, then resubmit the question separately and specify it. Thanks.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
