Question #8. Molecular Formula A certain compound has an empirical formula of C2H,OSi and a molecular weight of 593.236 amu. Determine the molecular formula of this compound. Show your numerical set-up(s) in the editing pane below. Use correct units and the correct number of significant figures in your set-up(s. Record your final answer in the next question.
Question #8. Molecular Formula A certain compound has an empirical formula of C2H,OSi and a molecular weight of 593.236 amu. Determine the molecular formula of this compound. Show your numerical set-up(s) in the editing pane below. Use correct units and the correct number of significant figures in your set-up(s. Record your final answer in the next question.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 120GQ: Write the molecular formula and calculate the molar mass for each of the molecules shown here. Which...
Related questions
Question
Both questions refer to each other
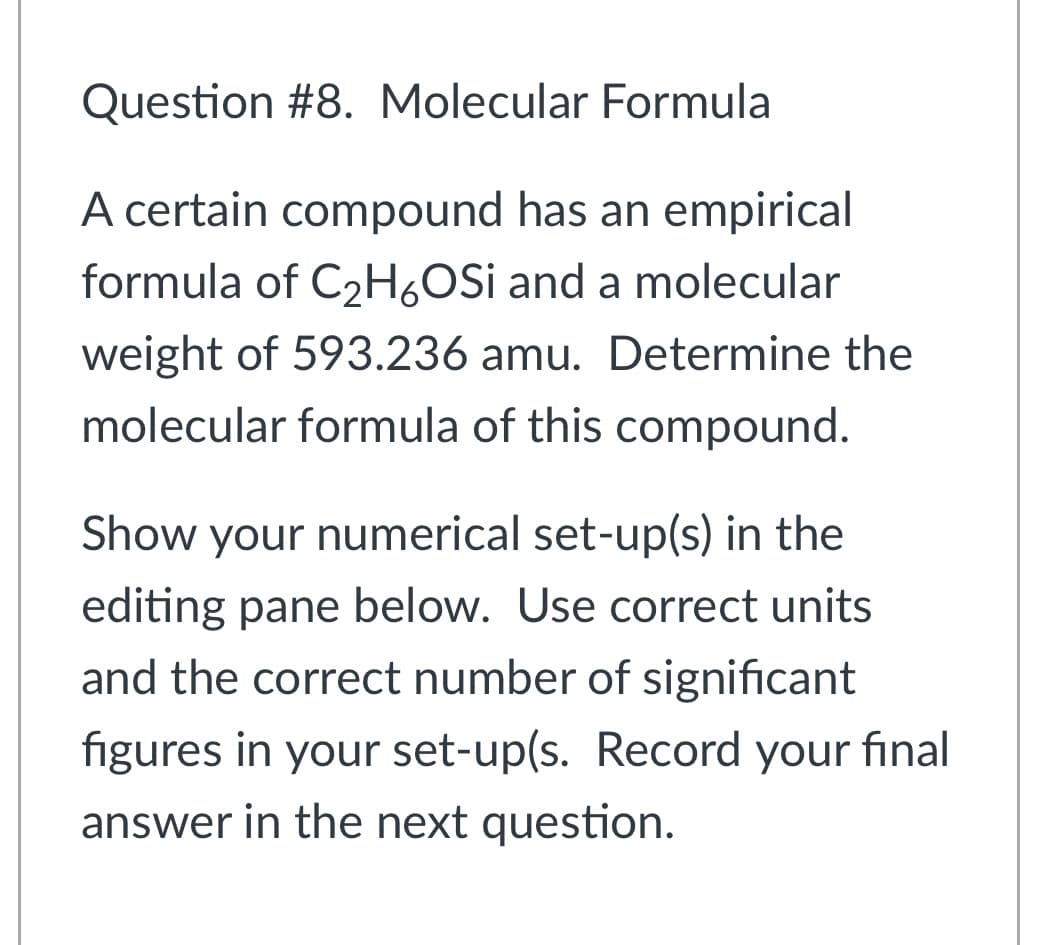
Transcribed Image Text:Question #8. Molecular Formula
A certain compound has an empirical
formula of C2H,OSi and a molecular
weight of 593.236 amu. Determine the
molecular formula of this compound.
Show your numerical set-up(s) in the
editing pane below. Use correct units
and the correct number of significant
figures in your set-up(s. Record your final
answer in the next question.
![Question #9. Answer for Question #8.
Record the molecular formula of the
compound in Question #8 in the box
below. Canvas will not allow you to enter
a subscript for a chemical formula; to
show subscripts, use the underscore. For
example, CaCl2 would be entered as
CaCl_2, N2S3 would be entered as
N_2S_3, and Al2(SO4)3 would be entered
as Al_2(SO_4)_3. In writing the molecular
formula, write the elements in the same
order in which they appear in the
empirical formula above.
[i]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fdd23d7d9-0699-49a5-8caf-6e827a8123cf%2F37efe080-e5ae-4c71-b341-fb8780086e13%2Ff0cg8ma_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Question #9. Answer for Question #8.
Record the molecular formula of the
compound in Question #8 in the box
below. Canvas will not allow you to enter
a subscript for a chemical formula; to
show subscripts, use the underscore. For
example, CaCl2 would be entered as
CaCl_2, N2S3 would be entered as
N_2S_3, and Al2(SO4)3 would be entered
as Al_2(SO_4)_3. In writing the molecular
formula, write the elements in the same
order in which they appear in the
empirical formula above.
[i]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning