Question: You have 10 kg of water at -15°C. You wish to heat it in an insulated box with a heater to a consumable temperature of 1°C. a. Show the thermodynamic calculation for this problem. b. How much energy would this require?
Question: You have 10 kg of water at -15°C. You wish to heat it in an insulated box with a heater to a consumable temperature of 1°C. a. Show the thermodynamic calculation for this problem. b. How much energy would this require?
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter2: Steady Heat Conduction
Section: Chapter Questions
Problem 2.39P: The tip of a soldering iron consists of a 0.6-cm- diameter copper rod, 7.6 cm long. If the tip must...
Related questions
Question
Show neat and clear work
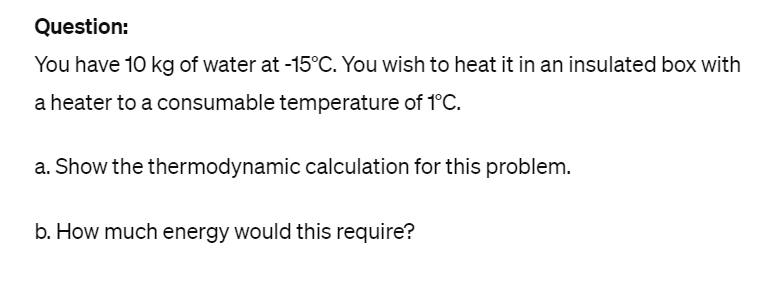
Transcribed Image Text:Question:
You have 10 kg of water at -15°C. You wish to heat it in an insulated box with
a heater to a consumable temperature of 1°C.
a. Show the thermodynamic calculation for this problem.
b. How much energy would this require?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning