Rank the following elements according to their ionization energy. element ionization energy germanium (Choose one) carbon (Choose one) v fludine (Choose one) v gallium (Choose one)
Rank the following elements according to their ionization energy. element ionization energy germanium (Choose one) carbon (Choose one) v fludine (Choose one) v gallium (Choose one)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 39P: Without consulting any tables, arrange the following substances in order and explain your choice of...
Related questions
Question
The first question I sent like that you guys gave me wrong answer , please make sure this is right
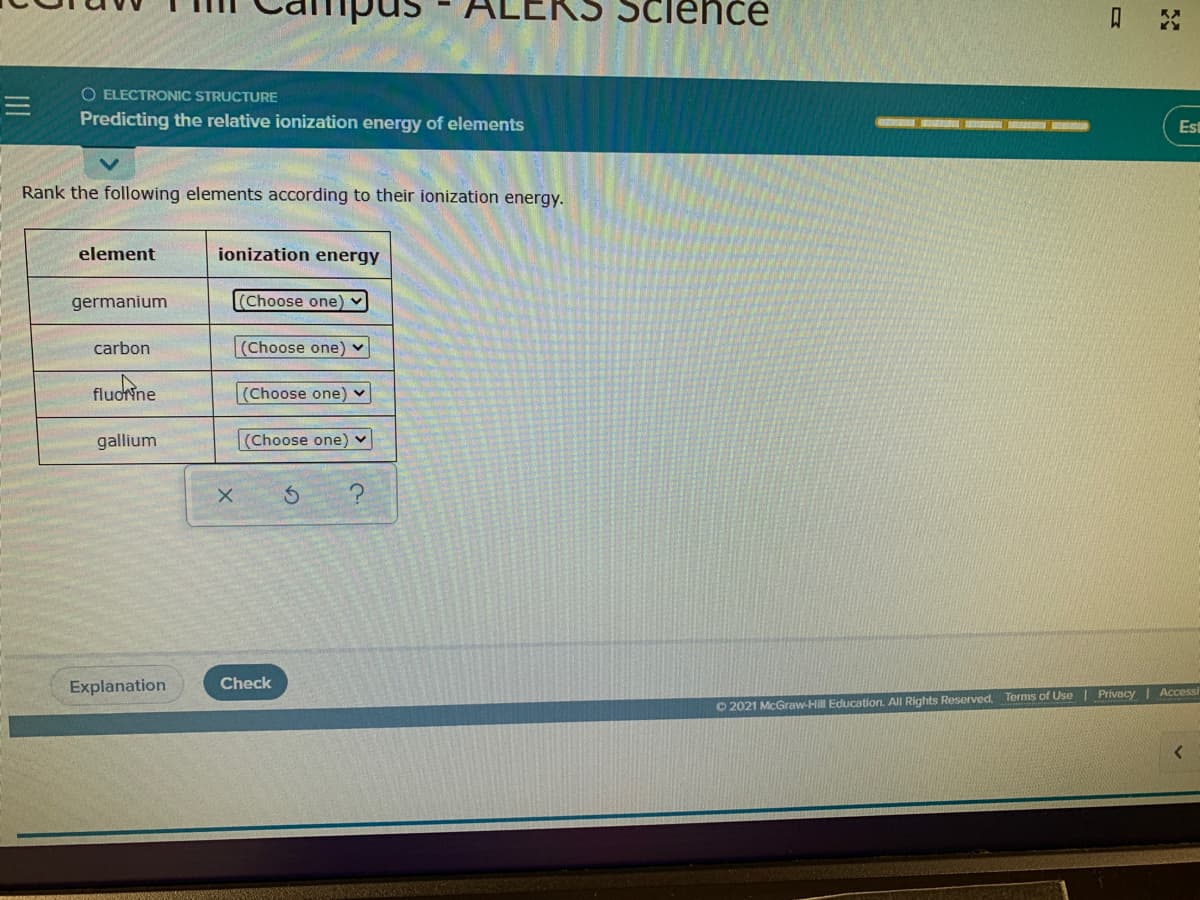
Transcribed Image Text:O ELECTRONIC STRUCTURE
Predicting the relative ionization energy of elements
Es
Rank the following elements according to their ionization energy.
element
ionization energy
germanium
(Choose one) v
carbon
(Choose one) v
fluoine
(Choose one)
gallium
(Choose one)
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy I Accessi
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co