Rank the ultraviolet, infrared, and visible regions of the electromagnetic spectrum in terms of lowest to highest wavelength, energy, and frequency.
Rank the ultraviolet, infrared, and visible regions of the electromagnetic spectrum in terms of lowest to highest wavelength, energy, and frequency.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 59AP
Related questions
Question
Q2
Transcribed Image Text:Ultraviolet
Visible
Infrared
5 1
4-1
3 1
2 1
4.
3
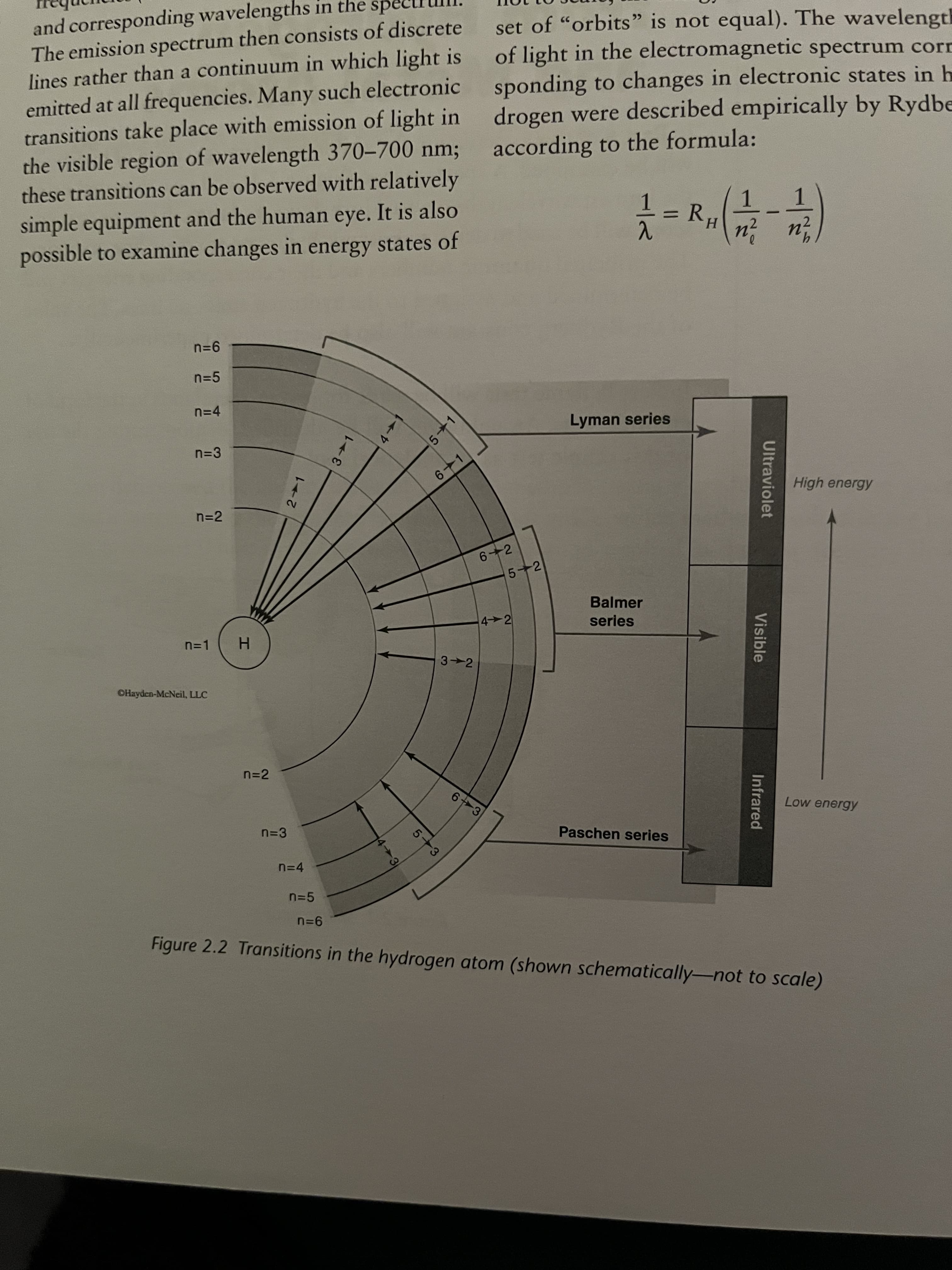
and corresponding wavelengths in the
The emission spectrum then consists of discrete
lines rather than a continuum in which light is
emitted at all frequencies. Many such electronic
transitions take place with emission of light in
the visible region of wavelength 370-700 nm;
these transitions can be observed with relatively
sp
set of "orbits" is not equal). The wavelength
of light in the electromagnetic spectrum corr
sponding to changes in electronic states in h
drogen were described empirically by Rydbe
according to the formula:
simple equipment and the human eye. It is also
possible to examine changes in energy states of
1.
R.
1.
1.
H.
n=5
n=4
Lyman series
n=3
6-1
High energy
n=2
6 2
5+2
Balmer
4 2
series
n=1
H.
3 2
OHayden-McNeil, LLC
n-2
6 3
Low energy
n=3
Paschen series
n=4
Figure 2.2 Transitions in the hydrogen atom (shown schematically-not to scale)

Transcribed Image Text:1. Rank the ultraviolet, infrared, and visible regions of the electromagnetic spectrum in terms
of lowest to highest wavelength, energy, and frequency.
Wavelength:
Energy:
Frequency:
2. Rank the following three transitions in the hydrogen atom in terms of lowest to highest wave-
length, energy, and frequency: 6 → 5, 3 → 1,7→ 3. Note that the energy levels in Figure 2.2
are shown schematically, so you must calculate the energy difference between any two levels
in order to answer this question. Show all calculations below or on the following page.
Wavelength:
Energy:
Frequency:
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
