Reaction 5: Balanced Reaction Equation: List of substanced added AND Amount of each to solution tube: substance added: Safety hazards to be aware of (give at least one!): Color of the resulting copper product: Write the Balanced Reaction equation for the reaction of H₂SO4 with solid Zinc:
Reaction 5: Balanced Reaction Equation: List of substanced added AND Amount of each to solution tube: substance added: Safety hazards to be aware of (give at least one!): Color of the resulting copper product: Write the Balanced Reaction equation for the reaction of H₂SO4 with solid Zinc:
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter7: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 10A
Related questions
Question
Reaction 5?
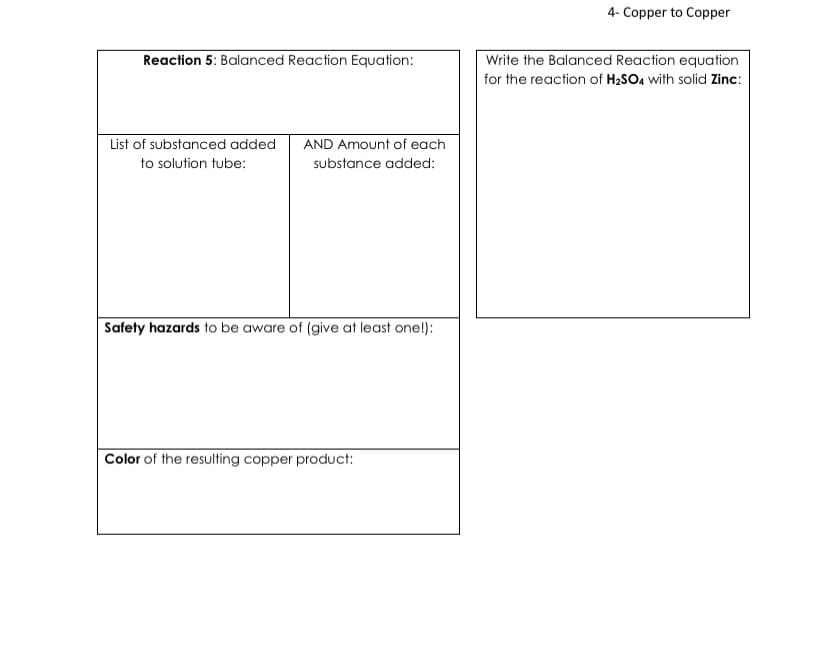
Transcribed Image Text:Reaction 5: Balanced Reaction Equation:
List of substanced added
to solution tube:
AND Amount of each
substance added:
Safety hazards to be aware of (give at least one!):
Color of the resulting copper product:
4- Copper to Copper
Write the Balanced Reaction equation
for the reaction of H₂SO4 with solid Zinc:
![Reactions copper (II) sulfate → copper]
1.
Transfer the reaction mixture into a clean 50 mL beaker.
2. All at once, add TWO measures of zinc granules to the reaction beaker. Definition: One measure
of zinc has a mass equal to the mass of copper you used in the first reaction. Don't spend too
much time getting the perfect amount. As long as the amount of zinc you weigh out is within +/-
0.02 grams, you'll be fine. Stir continuously using your glass rod. Do not use the rubber tip on the
glass as the copper will stick to it.
3. If any of the zinc sticks to the side of the tube above the liquid, use your stir rod to push it
beneath the surface of the liquid. The solution color should fade as solid copper forms.
4.
Decant the liquid into a new clean 50 mL beaker (be careful not to lose any copper). You should
now have two beakers: one beaker containing a "dirty" sample of solid copper, and one beaker
containing a pale blue solution.
5. Add ONE measure of zinc granules to the beaker containing the pale blue solution. If you see any
unreacted black zinc after the fizzing has stopped, add ~1.5 mL 6.0M H₂SO4 and wait until you see
no more fizzing. After the solution becomes colorless (it may not be completely colorless, but it
should not have a blue tint) and the bubbling of hydrogen gas has stopped, let the copper solid
settle to the bottom for two minutes. Carefully decant the liquid into a waste container.
5. You will now have two beakers containing "dirty" copper. Combine them into one beaker,
7. Wash the copper by rinsing with 5 mL of DI water and decanting the rinse water into a waste
beaker. Wash three more times, using 5 mL aliquots of water each time.
Record your observations.
3.
This reaction brings us back to the solid copper that the first reaction started with. Zinc sulfate is
also a product in this reaction, but it is soluble in water. (It was removed when you decanted the
liquid.)
copper(II) sulfate + zinc→ copper + zinc sulfate
PRELAB Assignment
Lab Day:
IMPORTANT DIRECTIONS: After reading the entire handout, complete the following diagrams of
the five reactions steps. The first one is done for you, as an example of what is expected. This is
due at the start of lab. You will NOT be allowed to complete this lab (at any time and will receive
a score of '0') without turning in this COMPLETED page at the start of lab.
Reaction 2: Balanced Reaction Equation
Reaction 1: Balanced Reaction Equation
Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H₂O
List of stand
added to centrifuge
tube:
1. Copper
2. 6M Nitric acid
3. water
Name:
AND Amount of
each substance
added:
15 cm x 2.5 cm
1.
2.
1.5 mL
3. Until tube is 2/3 full
Safety hazards to be aware of (give at least
one!):
1. Acid is corrosive
2. NO₂ gas is toxic, keep under Fume Hood!
Color of the copper product: blue solution
List of substances
added to centrifuge
tube:
Amount
each substance
added:
Safety hazards to be aware of (give at least
one!):
Color of the copper product:](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd407bb69-65e0-4600-bfd9-f9e537a98b1a%2F578afabe-926f-48f0-8275-9ee4c101864d%2Fxjjgab_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Reactions copper (II) sulfate → copper]
1.
Transfer the reaction mixture into a clean 50 mL beaker.
2. All at once, add TWO measures of zinc granules to the reaction beaker. Definition: One measure
of zinc has a mass equal to the mass of copper you used in the first reaction. Don't spend too
much time getting the perfect amount. As long as the amount of zinc you weigh out is within +/-
0.02 grams, you'll be fine. Stir continuously using your glass rod. Do not use the rubber tip on the
glass as the copper will stick to it.
3. If any of the zinc sticks to the side of the tube above the liquid, use your stir rod to push it
beneath the surface of the liquid. The solution color should fade as solid copper forms.
4.
Decant the liquid into a new clean 50 mL beaker (be careful not to lose any copper). You should
now have two beakers: one beaker containing a "dirty" sample of solid copper, and one beaker
containing a pale blue solution.
5. Add ONE measure of zinc granules to the beaker containing the pale blue solution. If you see any
unreacted black zinc after the fizzing has stopped, add ~1.5 mL 6.0M H₂SO4 and wait until you see
no more fizzing. After the solution becomes colorless (it may not be completely colorless, but it
should not have a blue tint) and the bubbling of hydrogen gas has stopped, let the copper solid
settle to the bottom for two minutes. Carefully decant the liquid into a waste container.
5. You will now have two beakers containing "dirty" copper. Combine them into one beaker,
7. Wash the copper by rinsing with 5 mL of DI water and decanting the rinse water into a waste
beaker. Wash three more times, using 5 mL aliquots of water each time.
Record your observations.
3.
This reaction brings us back to the solid copper that the first reaction started with. Zinc sulfate is
also a product in this reaction, but it is soluble in water. (It was removed when you decanted the
liquid.)
copper(II) sulfate + zinc→ copper + zinc sulfate
PRELAB Assignment
Lab Day:
IMPORTANT DIRECTIONS: After reading the entire handout, complete the following diagrams of
the five reactions steps. The first one is done for you, as an example of what is expected. This is
due at the start of lab. You will NOT be allowed to complete this lab (at any time and will receive
a score of '0') without turning in this COMPLETED page at the start of lab.
Reaction 2: Balanced Reaction Equation
Reaction 1: Balanced Reaction Equation
Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H₂O
List of stand
added to centrifuge
tube:
1. Copper
2. 6M Nitric acid
3. water
Name:
AND Amount of
each substance
added:
15 cm x 2.5 cm
1.
2.
1.5 mL
3. Until tube is 2/3 full
Safety hazards to be aware of (give at least
one!):
1. Acid is corrosive
2. NO₂ gas is toxic, keep under Fume Hood!
Color of the copper product: blue solution
List of substances
added to centrifuge
tube:
Amount
each substance
added:
Safety hazards to be aware of (give at least
one!):
Color of the copper product:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
