REACTION OF LIPIDS Results: Test Treatment Result Solubility of coconut oil in: a.Distilled water b.5% HCI c.5% NaOH d,diethyl.Ether e.Ethyl Alcohol f.Chloroform g.CCl, Translucent Coconut oil spot Glycerol Acrolein Test Coconut oil Glycerol Oleic acid Unsaturation Coconut oil Oleic acid Palmitic acid
REACTION OF LIPIDS Results: Test Treatment Result Solubility of coconut oil in: a.Distilled water b.5% HCI c.5% NaOH d,diethyl.Ether e.Ethyl Alcohol f.Chloroform g.CCl, Translucent Coconut oil spot Glycerol Acrolein Test Coconut oil Glycerol Oleic acid Unsaturation Coconut oil Oleic acid Palmitic acid
Chapter18: Vitamins, Minerals, And Herbals
Section: Chapter Questions
Problem 23RQ
Related questions
Question
Please help me answer the table

Transcribed Image Text:ACTIVITY NO 5
REACTION OF LIPIDS
Introduction
Lipids are heterogeneous class of bioorganic compounds which includes fats
and oils, waxes, cholesterol and its derivatives, some vitamins and prostaglandins.
What these substances have in common is their limited solubility in water.
Fats and oils are the most abundant lipids found in nature. Both are
triacylglycerol mixtures. Fats contain higher percentage of saturated fatty acid while
oils have more of the unsaturated fatty acids.
The chemical properties of triacylglycerol are typical with that of esters and
alkanes. Important reactions of these compounds are hydrolysis, saponification,
hydrogenation and rancidity and emulsification.
Hydrolysis may occur making them acidic giving rise to volatile fatty acid
which undergoes oxidation to give foul odor of volatile aldehydes and ketones..
Objectives: At the end of the activity, the student can:
1. List some chemical properties of lipids.
2. Identify the best solvents for lipids.
3.Test the different components of lipids.
4. Differentiate rancid from fresh oil using different indicators.
Reagents: 5 ml Coconut oil, 1 ml diethylether, 1 ml-5% HCI, 1 ml-5% NaOH, 1 ml
ethyl alcohol, 1 ml benzene, 1 ml CCl, 1 ml distilled water, 5 ml
chloroform, blue and red litmus paper, 3 drops phenolphthalein, 3 drops
methyl orange, few crystals KHS0,drops albumin, 1 g cholesterol,1 ml
acetic anhydride, 1.5 ml conc. H,SO, 1 ml glycerol, palmitic acid, 2 ml
oleic acid, 3 ml- 0.05M KMNO, solution, 3 ml-10% Na,CO,
BRING: bile solution, soap solution
Materials:10 ( 20ml Test tubes), test tube rack, test tube brush, test tube holder, 6
dropper, 10 ml graduated cylinder, evaporating dish, platform balance,
alcohol lamp, spatula
Procedure:
1. Solubility – Place 1 ml of distilled water, 5% HCl, 5% NaOH, diethyl ether, CCL,
ethyl alcohol, chloroform, into 7 different test tubes. Add 1 drop of coconut oil and
shake. Observe solubility of oil in the different solvents.
2. Translucent spots – Place 1 drop of coconut oil on a piece of ordinary writing
paper. Note the formation of a semi-transparent spot. Allow to evaporate
spontaneously. Observe what happens to the transparent spot. Repeat using
glycerol instead of coconut oil.
3. Acrolein Test – Place 5 drops of coconut oil in a test tube. Add a few crystals of
KHSO, and heat. Observe change in color and odor evolved. Repeat using
glycerol and oleic acid instead of coconut oil.
4. Test for Unsaturation – Prepare 3 test tube. To the first add 10 drops coconut oil,
to the second, add 10 drops oleic acid, the third a few crystals of palmitic acid.
Add 1 ml of 0.05M KMNO, and 1 ml of 10% Na.CO;. Shake the mixture and
allow to stand for a few minutes with occasional shaking. Observe the color of the
KMNO, layer.
5. Test for Rancidity – Prepare 3test tubes and add 5 drops of fresh coconut oil to
each tube. To the first tube , add 2 drops phenolphthalein, to the second 1 drop
methyl orange, and to the third place a piece of blue and red litmus paper. Repeat
using rancid oil instead of fresh coconut oil.
6. Emulsification – Prepare 3 test tubes and add 1 ml of coconut oil to each. To the
first tube, add 5 drops of bile solution, to the second 5 drops albumin and to the
third 5 drops soap solution. Shake each mixture very well and compare results.
7. Liebermann-Burchard Test – Dissolve 0.5 g of cholesterol in 2 ml chloroform and
place in an evaporating dish. Add 1 ml acetic anhydride and 5 drops of conc.
Sulfuric acid. Mix and place in the dark for 10 minutes. Observe color change.
8. Salkowski Test – Dissolve 0.5 g of cholesterol in 2 ml of chloroform. Allow 1 ml
of conc. Sulfuric acid to flow along the side of the tube avoiding the mixture with
chloroform layer. Note color produces both in the chloroform and sulfuric acid
layers.
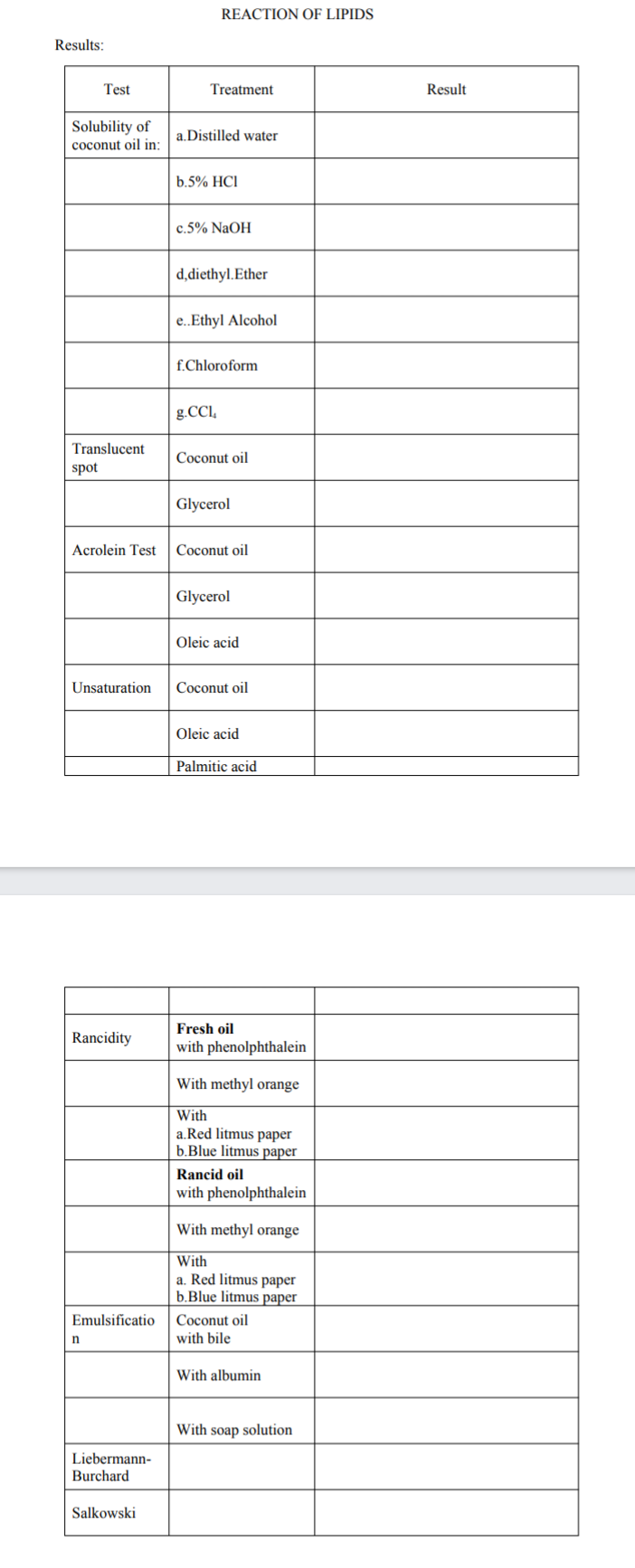
Transcribed Image Text:REACTION OF LIPIDS
Results:
Test
Treatment
Result
Solubility of
coconut oil in:
a.Distilled water
b.5% HCI
c.5% NaOH
d,diethyl.Ether
e.Ethyl Alcohol
f.Chloroform
g.CCl,
Translucent
Coconut oil
spot
Glycerol
Acrolein Test
Coconut oil
Glycerol
Oleic acid
Unsaturation
Coconut oil
Oleic acid
Palmitic acid
Fresh oil
Rancidity
with phenolphthalein
With methyl orange
With
a.Red litmus paper
b.Blue litmus paper
Rancid oil
with phenolphthalein
With methyl orange
With
a. Red litmus paper
b.Blue litmus paper
Emulsificatio
Coconut oil
with bile
With albumin
With soap solution
Liebermann-
Burchard
Salkowski
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning


Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning