H₂(g)+12(g) → 2 HI(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.2. P The engineer charges ("fills") three reaction vessels with hydrogen and iodine, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel A B с compound H₂ 1₂ HI H₂ 1₂ HI H₂ 1₂ HI pressure 2.39 atm 3.83 atm 4.71 atm. 2.82 atm. 4.26 atm 3.85 atm 3.81 atm. 5.76 atm 5.21 atm expected change in pressure Ot increase O decrease Ot increase O decrease Ot increase Ot increase Ot increase Ot increase O decrease O decrease Odecrease O decrease Ot increase O↑ increase O decrease decrease Ot increase O decrease X O(no change) (no change) (no change) (no change) (no change) (no change) O(no change) (no change) (no change) 5
H₂(g)+12(g) → 2 HI(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.2. P The engineer charges ("fills") three reaction vessels with hydrogen and iodine, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel A B с compound H₂ 1₂ HI H₂ 1₂ HI H₂ 1₂ HI pressure 2.39 atm 3.83 atm 4.71 atm. 2.82 atm. 4.26 atm 3.85 atm 3.81 atm. 5.76 atm 5.21 atm expected change in pressure Ot increase O decrease Ot increase O decrease Ot increase Ot increase Ot increase Ot increase O decrease O decrease Odecrease O decrease Ot increase O↑ increase O decrease decrease Ot increase O decrease X O(no change) (no change) (no change) (no change) (no change) (no change) O(no change) (no change) (no change) 5
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.41PAE: Because calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go...
Related questions
Question

Transcribed Image Text:A chemical engineer is studying the following reaction:
H₂(g)+I₂(g) → 2 HI(g)
At the temperature the engineer picks, the equilibrium constant
The engineer charges ("fills") three reaction vessels with hydrogen and iodine, and lets the reaction begin. He then measures the composition of the mixture
inside each vessel from time to time. His first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time he measures the compositions.
reaction
vessel
A
B
C
compound
H₂
1₂
HI
H₂
1₂
HI
H₂
1₂
HI
pressure
2.39 atm
3.83 atm
4.71 atm
2.82 atm
4.26 atm
3.85 atm
3.81 atm
5.76 atm
5.21 atm
expected change in pressure
↑ increase
increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
for this reaction is 1.2.
р
↑ increase
↑ increase
↓ decrease
↓decrease
↓ decrease
↓ decrease
↓decrease
↓ decrease
↓ decrease
↓decrease
↓ decrease
X
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
Ś
Expert Solution
Step 1
The reaction quotient Q is a measure of the relative amounts of products and reactants present in a reaction at a given time.
If Q < Kp , reaction proceeds to product side
If Q > Kp , reaction proceeds to reactants side
At equilibrium , Q = Kp
Given Kp = 1.2
Step by step
Solved in 4 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
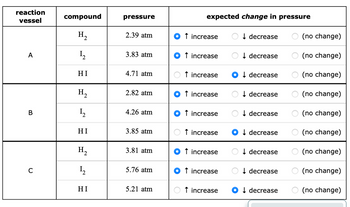
Transcribed Image Text:reaction
vessel
A
B
C
compound
H₂
1₂2
HI
H₂
1₂
HI
H₂
1₂2
HI
pressure
2.39 atm
3.83 atm
4.71 atm
2.82 atm
4.26 atm
3.85 atm
3.81 atm
5.76 atm
5.21 atm
expected change in pressure
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↓ decrease
↓ decrease
↓ decrease
↓ decrease
↓ decrease
Odecrease
↓ decrease
↓ decrease
↓ decrease
OO
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)

Transcribed Image Text:Try Again
Your answer is incorrect.
• reaction vessel B: Your answer is incorrect.
• reaction vessel C: Your answer is incorrect.
A chemical engineer is studying the following reaction:
H₂(g) + I₂(g) → 2 HI(g)
At the temperature the engineer picks, the equilibrium constant Kp for this reaction is 1.2.
р
The engineer charges ("fills") three reaction vessels with hydrogen and iodine, and lets the reaction begin. He then measures the composition of the mixture
inside each vessel from time to time. His first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time he measures the compositions.
reaction
vessel
A
B
compound
H₂
1₂
HI
H₂
1₂
HI
pressure
2.39 atm
3.83 atm
4.71 atm
2.82 atm
4.26 atm
3.85 atm
expected change in pressure
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↑ increase
↓ decrease
↓ decrease
↓decrease
↓ decrease
↓ decrease
↓ decrease
(no change)
(no change)
(no change)
(no change)
(no change)
(no change)
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning