Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. Change Is this change spontaneous? Yes. A solid substance dissolves in water, releasing heat as it does so. O No. Can't decide with information given. Yes. A solid precipitates from a solution, absorbing heat as it does so. O No. O Can't decide with information given. Yes. An endothermic chemical reaction between two liquids results in gaseous products. O No. O Can't decide with information given.
Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. Change Is this change spontaneous? Yes. A solid substance dissolves in water, releasing heat as it does so. O No. Can't decide with information given. Yes. A solid precipitates from a solution, absorbing heat as it does so. O No. O Can't decide with information given. Yes. An endothermic chemical reaction between two liquids results in gaseous products. O No. O Can't decide with information given.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.101PAE
Related questions
Question
100%
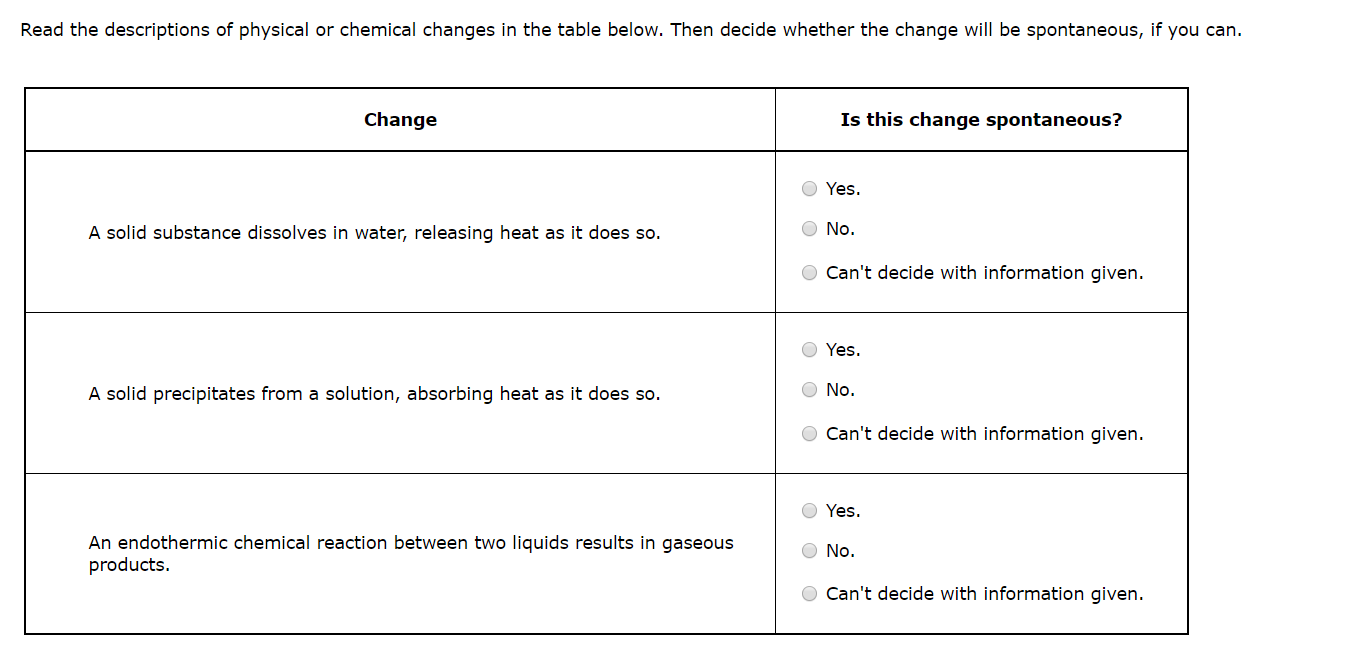
Transcribed Image Text:Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can.
Change
Is this change spontaneous?
Yes.
A solid substance dissolves in water, releasing heat as it does so.
O No.
Can't decide with information given.
Yes.
A solid precipitates from a solution, absorbing heat as it does so.
O No.
O Can't decide with information given.
Yes.
An endothermic chemical reaction between two liquids results in gaseous
products.
O No.
O Can't decide with information given.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
