Recognizing consistency between statements about standard G... The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to it. Otherwise, check the "no false statements" box under the table. false? statement false? statement ? X In K< O In K 1 ΔΗ9 > TΔS . ΔΗ. ΤΔSo K< 1 K 1 AG O AG 0 no false statements: no false statements: Explanation Check O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Lenovo PA IX Esc X Del Home Insert End FnLock F2 F4 F1 F3 F8 F5 F6 F7 F11 F12 F9 F10 E & Ba 4 3 6 2 5 7 II CO LO
Recognizing consistency between statements about standard G... The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to it. Otherwise, check the "no false statements" box under the table. false? statement false? statement ? X In K< O In K 1 ΔΗ9 > TΔS . ΔΗ. ΤΔSo K< 1 K 1 AG O AG 0 no false statements: no false statements: Explanation Check O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Lenovo PA IX Esc X Del Home Insert End FnLock F2 F4 F1 F3 F8 F5 F6 F7 F11 F12 F9 F10 E & Ba 4 3 6 2 5 7 II CO LO
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 16.DCP
Related questions
Question
100%
And each table there may be one statement that is false because it contradicts the other three statements if you find a false statement check the box next to it otherwise check the no-fault state in box under the table
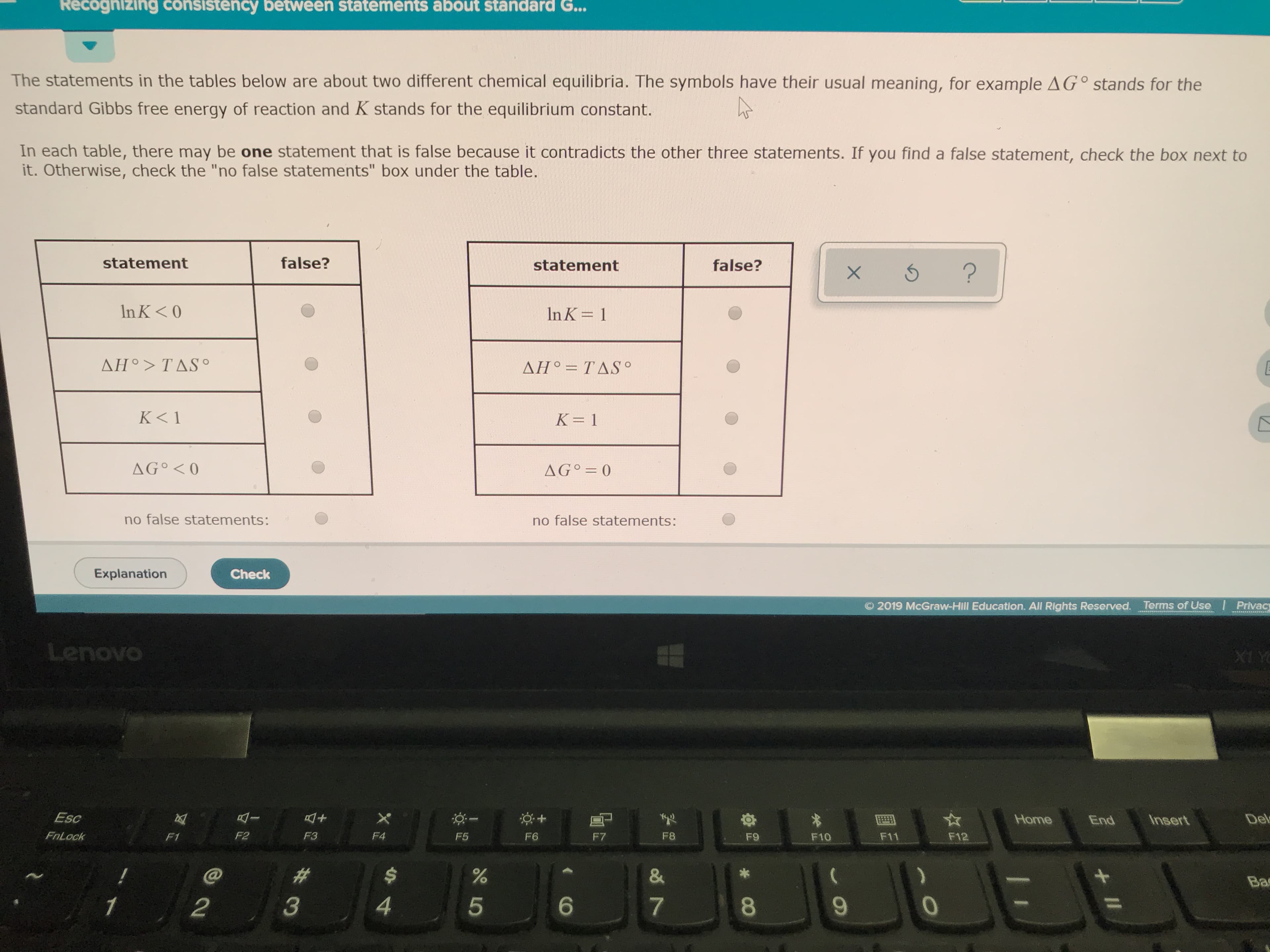
Transcribed Image Text:Recognizing consistency between statements about standard G...
The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the
standard Gibbs free energy of reaction and K stands for the equilibrium constant.
In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to
it. Otherwise, check the "no false statements" box under the table.
false?
statement
false?
statement
?
X
In K< O
In K 1
ΔΗ9 > TΔS .
ΔΗ. ΤΔSo
K< 1
K 1
AG O
AG 0
no false statements:
no false statements:
Explanation
Check
O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy
Lenovo
PA IX
Esc
X
Del
Home
Insert
End
FnLock
F2
F4
F1
F3
F8
F5
F6
F7
F11
F12
F9
F10
E
&
Ba
4
3
6
2
5
7
II
CO
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning