A 10.0-ml sample of aqueous NaOCI is treated with excess KI in an acidic solution. The quantity of iodine that is liberated is such that 28.02 mL of 0.0250 M Na2S₂O, solution must be added to cause the disappearance of the dark blue color due to the starch indicator. What is the molarity of the solution of NaOCI?
A 10.0-ml sample of aqueous NaOCI is treated with excess KI in an acidic solution. The quantity of iodine that is liberated is such that 28.02 mL of 0.0250 M Na2S₂O, solution must be added to cause the disappearance of the dark blue color due to the starch indicator. What is the molarity of the solution of NaOCI?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 20Q
Related questions
Question
Redo the third problem of the Prelaboratory Assignment, substituting NaIO3 (sodium iodate) for NaOCl. The new reaction yields I2 as the only product containing a halogen.
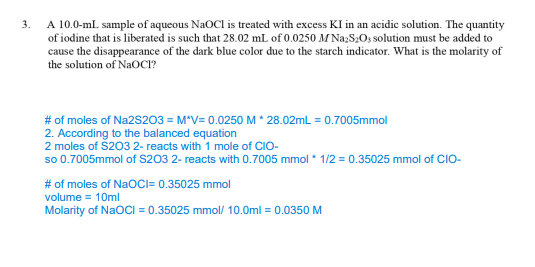
Transcribed Image Text:3.
A 10.0-mL sample of aqueous NaOCI is treated with excess KI in an acidic solution. The quantity
of iodine that is liberated is such that 28.02 mL of 0.0250 M Na₂S₂O3 solution must be added to
cause the disappearance of the dark blue color due to the starch indicator. What is the molarity of
the solution of NaOCI?
# of moles of Na2S203 = M*V= 0.0250 M * 28.02mL = 0.7005mmol
2. According to the balanced equation
2 moles of $203 2- reacts with 1 mole of CIO-
so 0.7005mmol of $203 2- reacts with 0.7005 mmol * 1/2 = 0.35025 mmol of CIO-
# of moles of NaOCI= 0.35025 mmol
volume = 10ml
Molarity of NaOCI=0.35025 mmol/ 10.0ml = 0.0350 M

Transcribed Image Text:The Strength of a Laundry Bleach
Prelaboratory Assignment
Write the balanced equations for the following oxidation-reduction reactions that you will
encounter in this experiment:
The reduction of CIO by I in acidic solution
1.
2.
a.
C1012H₂0+21Iz+CI+H₂O
b.
The reduction of I₂ by S₂O3 in acidic solution
2-
1₂+ 25 ₂0²² → 21+ Sad
What conversion factor will enable you to calculate the number of moles of ClO ions from the
number of moles of S₂O3 ions used in the titration?
2-
2 moles of $₂03 = 1 mole of
CIÓ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Redo the third problem of the Prelaboratory Assignment, substituting NaIO3 (sodium iodate) for NaOCl. The new reaction yields I2 as the only product containing a halogen?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
