Refrigerant 22 in a refrigeration system enters one side of a counter-flow heat exchanger at 12 bar, 28°C. The refrigerant exits at 12 bar, 20°C. A separate stream of R-22 enters the other side of the heat exchanger as saturated vapor at 2 bar and exits as superheated vapor at 2 bar. The mass flow rates of the two streams are equal. Stray heat transfer from the heat exchanger to its surroundings and kinetic and potential energy effects are negligible. Determine the entropy production in the heat exchanger, in kJ/K per kg of refrigerant flowing. What gives rise to the entropy production in this application? Show state point 1 and 2, then 3 and 4 on a T-s diagram, show the process from 1 to 2 and from 3 to 4.
Refrigerant 22 in a refrigeration system enters one side of a counter-flow heat exchanger at 12 bar, 28°C. The refrigerant exits at 12 bar, 20°C. A separate stream of R-22 enters the other side of the heat exchanger as saturated vapor at 2 bar and exits as superheated vapor at 2 bar. The mass flow rates of the two streams are equal. Stray heat transfer from the heat exchanger to its surroundings and kinetic and potential energy effects are negligible. Determine the entropy production in the heat exchanger, in kJ/K per kg of refrigerant flowing. What gives rise to the entropy production in this application? Show state point 1 and 2, then 3 and 4 on a T-s diagram, show the process from 1 to 2 and from 3 to 4.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
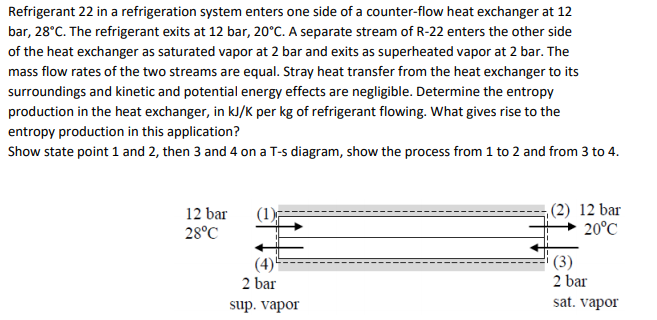
Transcribed Image Text:Refrigerant 22 in a refrigeration system enters one side of a counter-flow heat exchanger at 12
bar, 28°C. The refrigerant exits at 12 bar, 20°C. A separate stream of R-22 enters the other side
of the heat exchanger as saturated vapor at 2 bar and exits as superheated vapor at 2 bar. The
mass flow rates of the two streams are equal. Stray heat transfer from the heat exchanger to its
surroundings and kinetic and potential energy effects are negligible. Determine the entropy
production in the heat exchanger, in kJ/K per kg of refrigerant flowing. What gives rise to the
entropy production in this application?
Show state point 1 and 2, then 3 and 4 on a T-s diagram, show the process from 1 to 2 and from 3 to 4.
,(2) 12 bar
20°C
12 bar
(1)
28°C
(4)
2 bar
(3)
2 bar
sup. vapor
sat. vapor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY