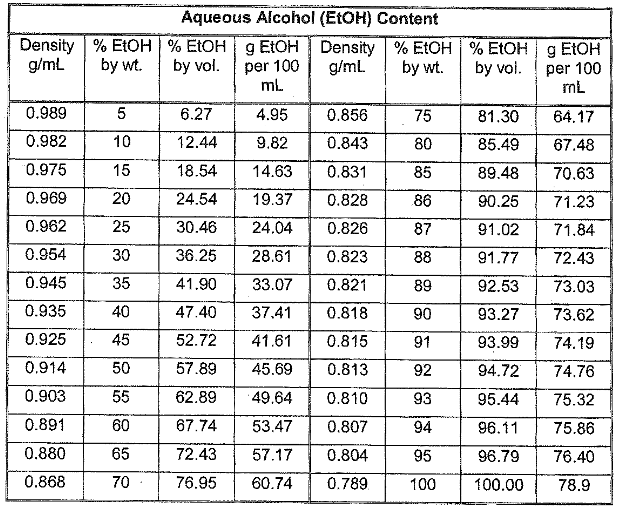
Report your results including: a) volume collected and % ethanol for each fraction b) % yield of ethanol from molasses c) % yield ethanol from sugars, assuming approximately half of molasses is reactive sugars. Show at least one representative calculation for each part (a, b, and c). The relevant reaction is: C12H22O11 (sucrose) -> 4 CH3CH2OH + 4 CO2. Data: Fraction 1: 1mL ethanol collected, 0.81g Fraction 2L 1mL ethanol collected, 0.95g Fraction 3: 1mL ethanol product collected, 0.97g
Report your results including: a) volume collected and % ethanol for each fraction b) % yield of ethanol from molasses c) % yield ethanol from sugars, assuming approximately half of molasses is reactive sugars. Show at least one representative calculation for each part (a, b, and c). The relevant reaction is: C12H22O11 (sucrose) -> 4 CH3CH2OH + 4 CO2. Data: Fraction 1: 1mL ethanol collected, 0.81g Fraction 2L 1mL ethanol collected, 0.95g Fraction 3: 1mL ethanol product collected, 0.97g
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.44E
Related questions
Question
Report your results including:
a) volume collected and % ethanol for each fraction
b) % yield of ethanol from molasses
c) % yield ethanol from sugars, assuming approximately half of molasses is reactive sugars.
Show at least one representative calculation for each part (a, b, and c).
The relevant reaction is: C12H22O11 (sucrose) -> 4 CH3CH2OH + 4 CO2.
Data:
Fraction 1: 1mL ethanol collected, 0.81g
Fraction 2L 1mL ethanol collected, 0.95g
Fraction 3: 1mL ethanol product collected, 0.97g
Transcribed Image Text:Density % EtOH
g/mL
by wt.
0.989
0.982
0.975
0.969
0.962
0.954
0.945
0.935
0.925
0.914
0.903
0.891
0.880
0.868
5
10
15
20
25
30
35
40
45
50
55
60
65
70
Aqueous Alcohol (EtOH) Content
% EtOH
g EtOH
Density
by vol.
per 100
g/mL.
mL.
6.27
4.95
0.856
12.44
9.82
0.843
18.54
14.63
0.831
24.54
19.37
0.828
30.46
24.04
0.826
36.25
28.61
0.823
41.90
33.07
0.821
47.40 37,41
0.818
52.72 41.61
0.815
57.89
45.69
0.813
62.89 49.64 0.810
67.74
53.47
0.807
72.43
57.17
0.804
76.95 60.74
0.789
% EtOH
by wt.
75
80
85
86
87
88
89
90
91
92
93
94
95
100
% EtOH g EtOH
by voi.
per 100
mL
81.30
64.17
85.49
67.48
89.48
70.63
90.25
71.23
91.02
71.84
91.77 72.43
92.53 73.03
93.27
73.62
93.99
74.19
94.72
74.76
95.44 75.32
96.11
75.86
96.79
76.40
100.00
78.9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
