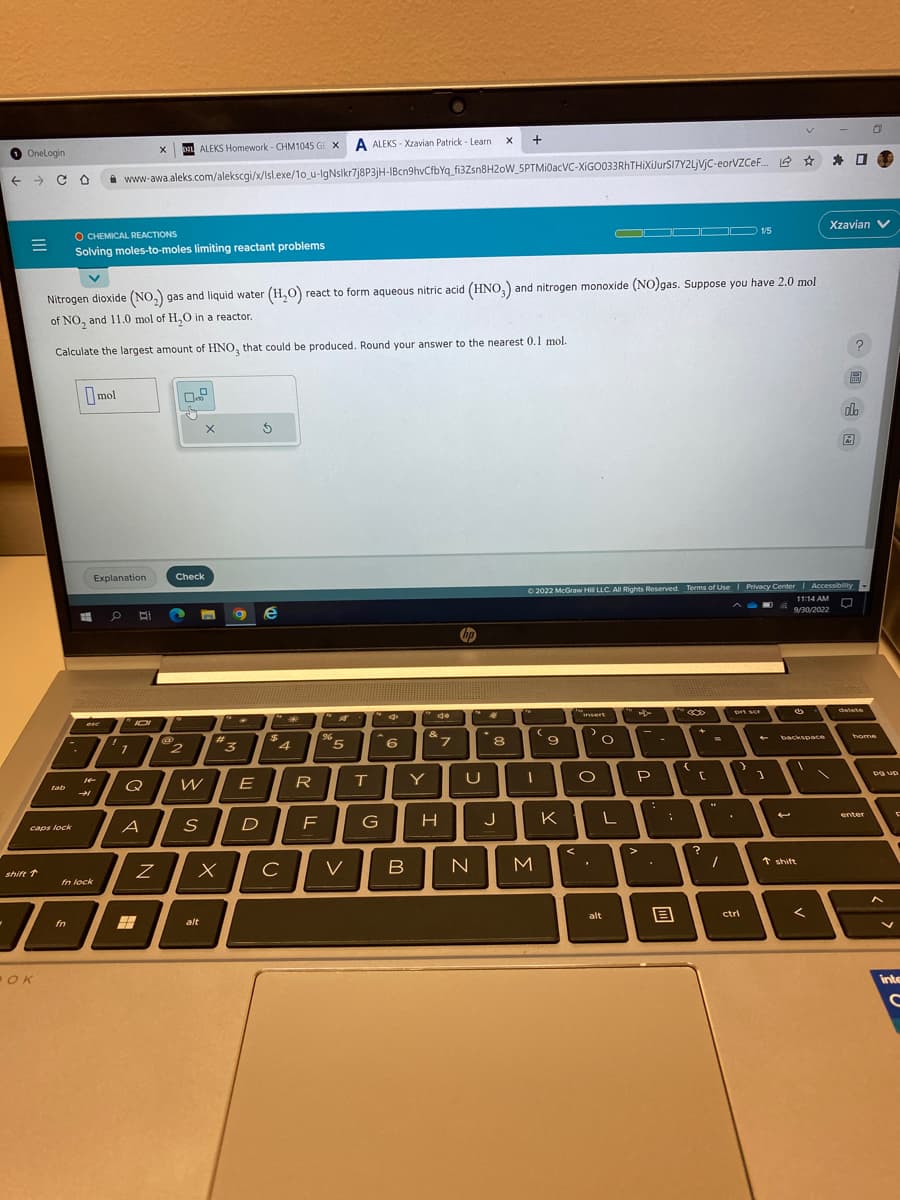
Nitrogen dioxide (NO₂) gas and liquid water (H₂O) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO)gas. Suppose you have 2.0 mol of NO₂ and 11.0 mol of H₂O in a reactor. Calculate the largest amount of HNO3 that could be produced. Round your answer to the nearest 0.1 mol. O mol d
Nitrogen dioxide (NO₂) gas and liquid water (H₂O) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO)gas. Suppose you have 2.0 mol of NO₂ and 11.0 mol of H₂O in a reactor. Calculate the largest amount of HNO3 that could be produced. Round your answer to the nearest 0.1 mol. O mol d
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 45QAP: For each of the following unbalanced reactions, suppose exactly 5.00 g of each reactant is taken....
Related questions
Question
Calculate the largest amount of HNO3 that could be produced. Round your answer to the nearest 0.1 mol
Transcribed Image Text:OneLogin
← CO
=
shift 1
tab
caps lock
OK
-
O CHEMICAL REACTIONS
Solving moles-to-moles limiting reactant problems
T
fn
V
Nitrogen dioxide (NO₂) gas and liquid water (H₂O) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO)gas. Suppose you have 2.0 mol
of NO, and 11.0 mol of H₂O in a reactor.
Calculate the largest amount of HNO, that could be produced. Round your answer to the nearest 0.1 mol.
➜
mol
esc
fn lock
Explanation
X DEL ALEKS Homework- CHM1045 GEX A ALEKS-Xzavian Patrick - Learn
www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H20W_5PTMI0acVC-XIGO033RhTHIXiJurSI7Y2LjVjC-eorVZCF... ✰ ✰
*
O B
"JOI
'1
Q
A
Z
@
Check
2
x
W
S
alt
#
X
a e
3
E
$
D
$
4
с
R
F
%6
5
V
T
G
6
Y
do
&
*7
H
U
X +
B N
8
J
C
(
I
M
9
Ⓒ2022 McGraw Hilll LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
11:14 AM
9/30/2022
K
<
insert
>
O
O
L
alt
P
>
-
:
B
{
+
C
?
=
prt sor
1/5
}
ctrl
1
(5
V
backspace
V
↑ shift
<
Xzavian V
圖
?
do
&
delete
home
enter
pa up
E
F
inte
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning