Required information NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Five kilograms of air at 427°C and 600 kPa are contained in a piston-cylinder device. The air expands adiabatically unti the pressure is 100 kPa and produces 450 kJ of work output. Assume air has constant specific heats evaluated at 300 k Determine the entropy change of the air in kJ/kg-K. Use the table containing the ideal gas specific heats of various common ga (You must provide an answer before moving on to the next part.) The entropy change of the air is 1.5 kJ/kg-K.
Required information NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Five kilograms of air at 427°C and 600 kPa are contained in a piston-cylinder device. The air expands adiabatically unti the pressure is 100 kPa and produces 450 kJ of work output. Assume air has constant specific heats evaluated at 300 k Determine the entropy change of the air in kJ/kg-K. Use the table containing the ideal gas specific heats of various common ga (You must provide an answer before moving on to the next part.) The entropy change of the air is 1.5 kJ/kg-K.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter1: Basic Modes Of Heat Transfer
Section: Chapter Questions
Problem 1.22P: 1.22 In order to prevent frostbite to skiers on chair lifts, the weather report at most ski areas...
Related questions
Question
Help with this problem
Transcribed Image Text:1:20
W
Check my work mode: This shows what is correct or incorrect for the work you have completed so far. It does not indicat
ere to search
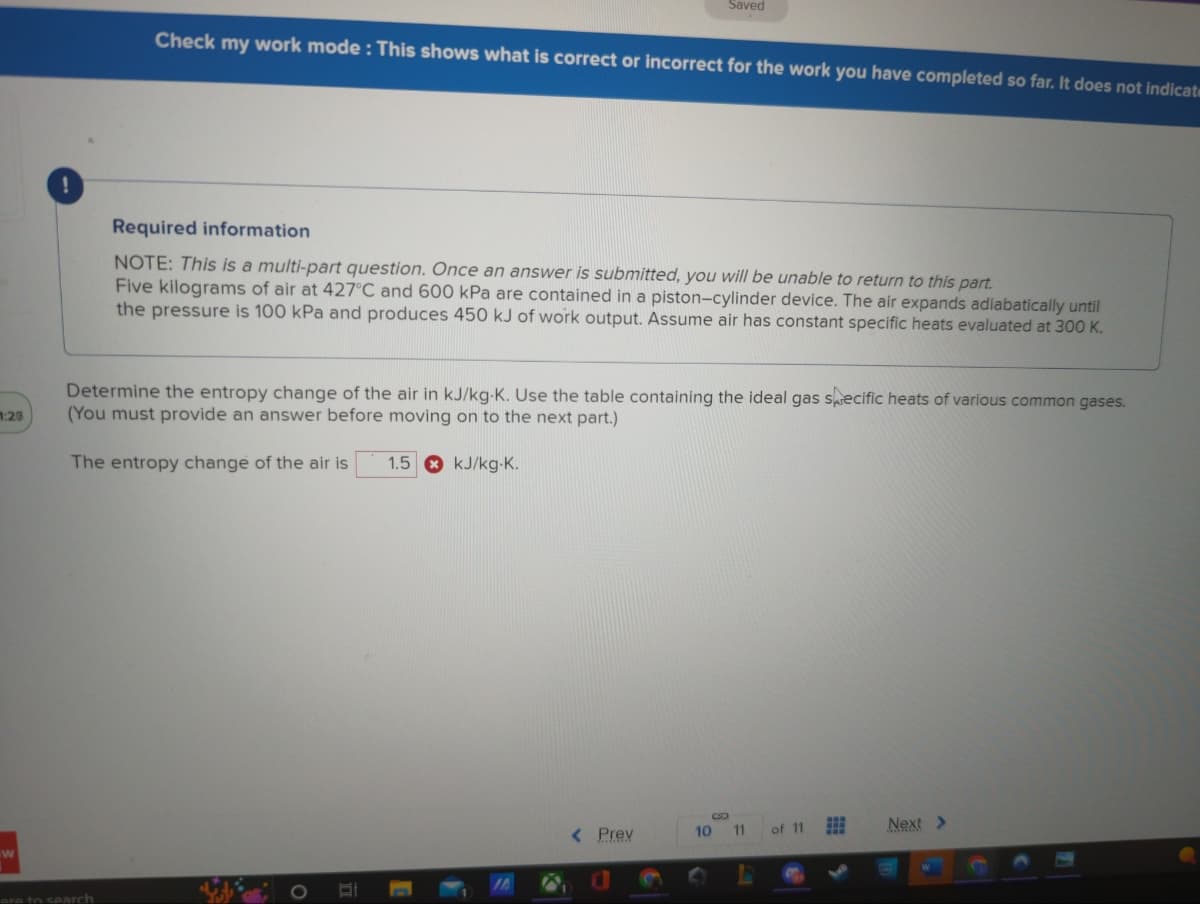
Required information
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
Five kilograms of air at 427°C and 600 kPa are contained in a piston-cylinder device. The air expands adiabatically until
the pressure is 100 kPa and produces 450 kJ of work output. Assume air has constant specific heats evaluated at 300 K.
Determine the entropy change of the air in kJ/kg-K. Use the table containing the ideal gas specific heats of various common gases.
(You must provide an answer before moving on to the next part.)
The entropy change of the air is
1.5
Saved
kJ/kg-K.
< Prev
10
11 of 11 ⠀⠀
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning