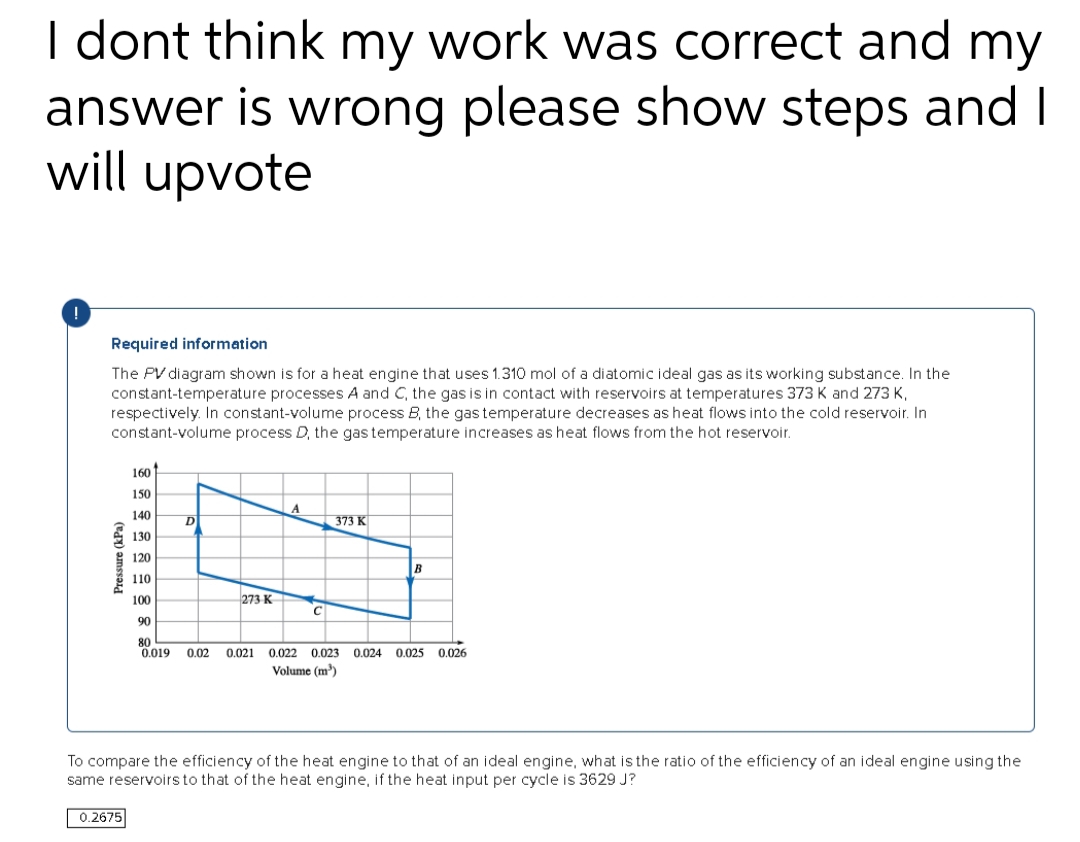
Required information The PV diagram shown is for a heat engine that uses 1.310 mol of a diatomic ideal gas as its working substance. In the constant-temperature processes A and C, the gas is in contact with reservoirs at temperatures 373 K and 273 K, respectively. In constant-volume process B, the gas temperature decreases as heat flows into the cold reservoir. In constant-volume process D, the gas temperature increases as heat flows from the hot reservoir. Pressure (kPa) 160 150 140 130 120 110 100 90 80 0.019 0.02 0.021 0.022 0.023 0.024 0.025 0.026 Volume (m³) D 273 K A 373 K B To compare the efficiency of the heat engine to that of an ideal engine, what is the ratio of the efficiency of an ideal engine using the same reservoirs to that of the heat engine, if the heat input per cycle is 3629 J?
Required information The PV diagram shown is for a heat engine that uses 1.310 mol of a diatomic ideal gas as its working substance. In the constant-temperature processes A and C, the gas is in contact with reservoirs at temperatures 373 K and 273 K, respectively. In constant-volume process B, the gas temperature decreases as heat flows into the cold reservoir. In constant-volume process D, the gas temperature increases as heat flows from the hot reservoir. Pressure (kPa) 160 150 140 130 120 110 100 90 80 0.019 0.02 0.021 0.022 0.023 0.024 0.025 0.026 Volume (m³) D 273 K A 373 K B To compare the efficiency of the heat engine to that of an ideal engine, what is the ratio of the efficiency of an ideal engine using the same reservoirs to that of the heat engine, if the heat input per cycle is 3629 J?
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter2: Steady Heat Conduction
Section: Chapter Questions
Problem 2.39P: The tip of a soldering iron consists of a 0.6-cm- diameter copper rod, 7.6 cm long. If the tip must...
Related questions
Question
100%
I vll upvote, handwritten acceptable
Transcribed Image Text:I dont think my work was correct and my
answer is wrong please show steps and I
will upvote
Required information
The PV diagram shown is for a heat engine that uses 1.310 mol of a diatomic ideal gas as its working substance. In the
constant-temperature processes A and C, the gas is in contact with reservoirs at temperatures 373 K and 273 K,
respectively. In constant-volume process B, the gas temperature decreases as heat flows into the cold reservoir. In
constant-volume process D, the gas temperature increases as heat flows from the hot reservoir.
Pressure (kPa)
160
150
140
130
120
110
100
90
80
0.019 0.02 0.021 0.022 0.023 0.024 0.025
Volume (m³)
0.2675
D
273 K
A
C
373 K
B
0.026
To compare the efficiency of the heat engine to that of an ideal engine, what is the ratio of the efficiency of an ideal engine using the
same reservoirs to that of the heat engine, if the heat input per cycle is 3629 J?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning