Revie Constam Part A The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3H2 (g) + N2 (g)-+2NH3 (g) What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. HA 10.9 圓
Revie Constam Part A The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3H2 (g) + N2 (g)-+2NH3 (g) What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. HA 10.9 圓
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.73PAE: 1.73 Why are two separate ITO layers required in a touch screen display?
Related questions
Question
Transcribed Image Text:A Maps
stry
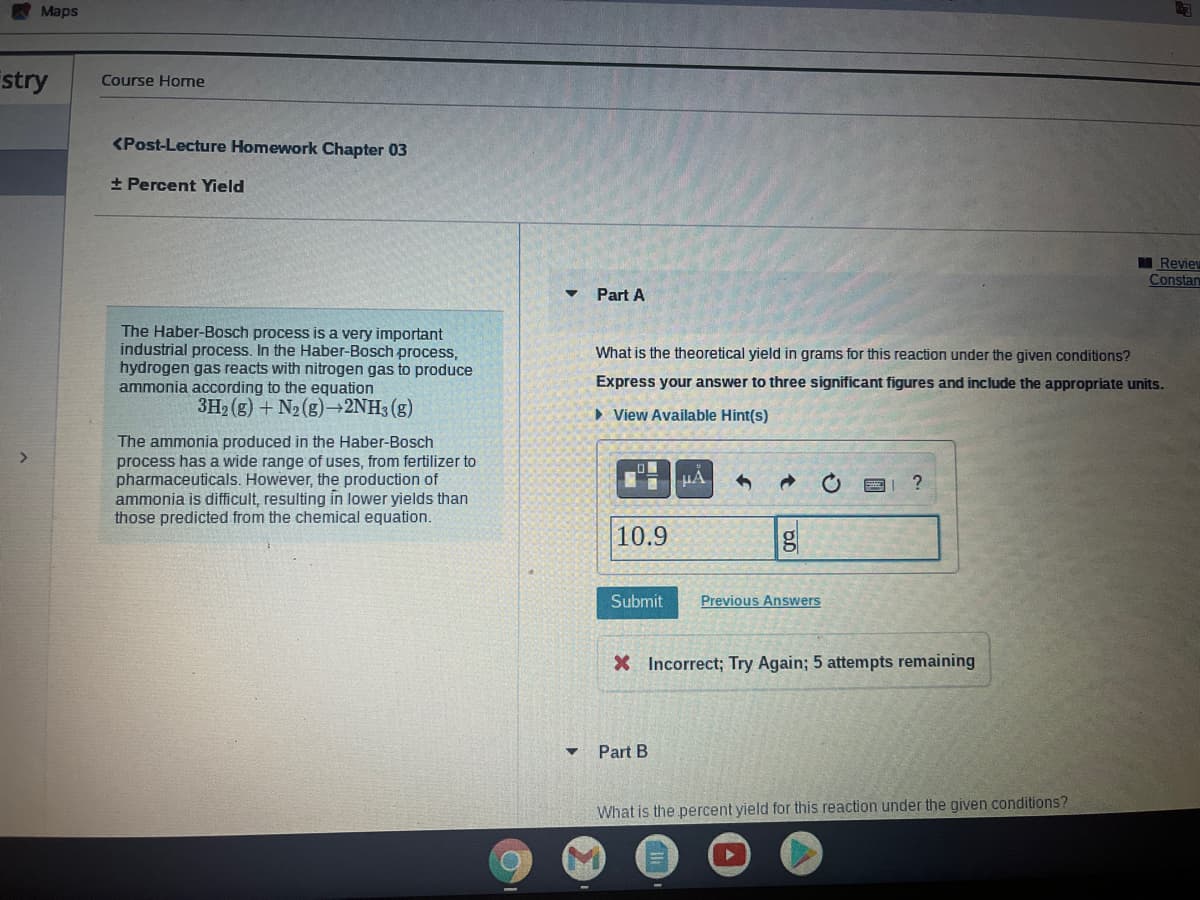
Course Home
<Post-Lecture Homework Chapter 03
+ Percent Yield
Review
Constan
Part A
The Haber-Bosch process is a very important
industrial process. In the Haber-Bosch process,
hydrogen gas reacts with nitrogen gas to produce
ammonia according to the equation
3H2 (g) + N2 (8) 2NH3 (g)
What is the theoretical yield in grams for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
The ammonia produced in the Haber-Bosch
process has a wide
pharmaceuticals. However, the production of
ammonia is difficult, resulting in lower yields than
those predicted from the chemical equation.
of uses, from fertilizer to
HA
10.9
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Part B
What is the percent yield for this reaction under the given conditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning