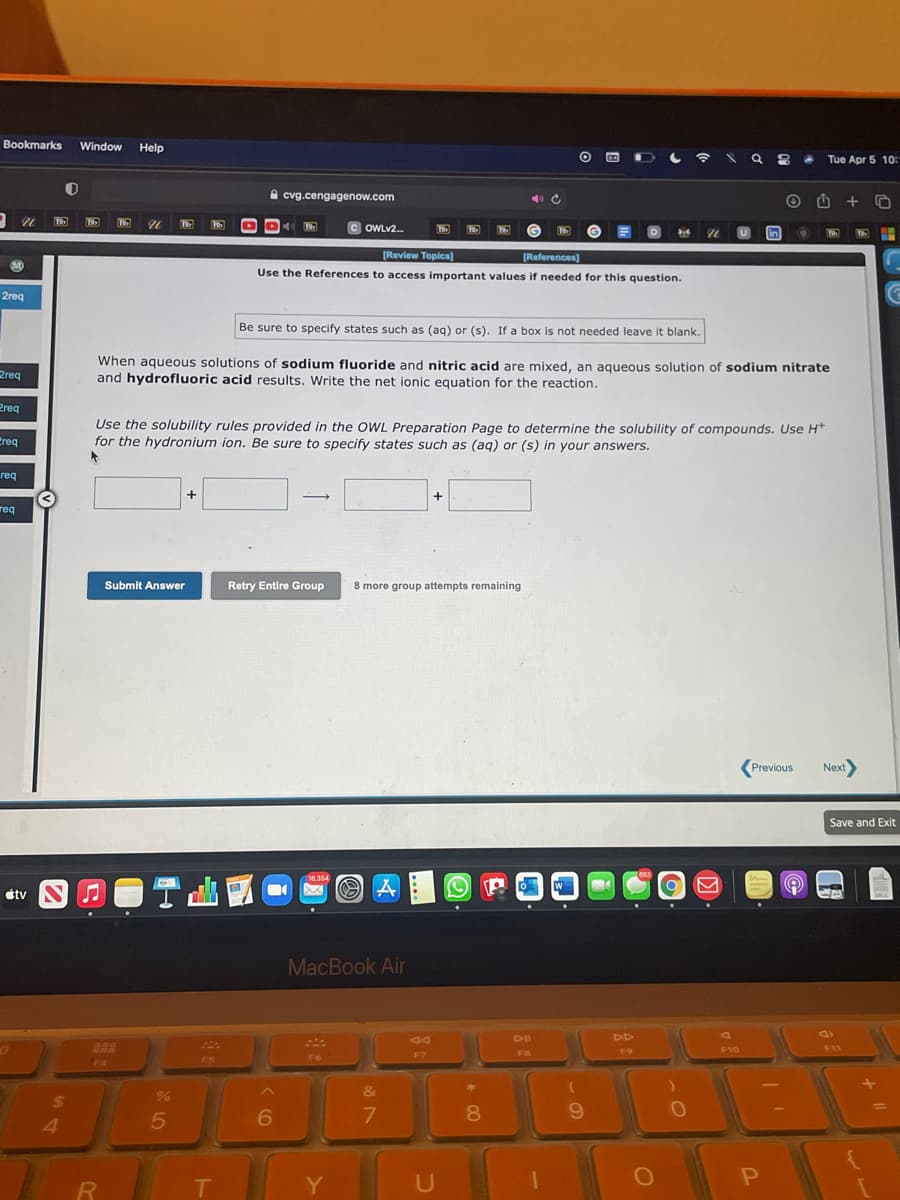
[Review Topica) [References) Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. When aqueous solutions of sodium fluoride and nitric acid are mixed, an aqueous solution of sodium nitrate and hydrofluoric acid results. Write the net ionic equation for the reaction. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Use H* for the hydronium ion. Be sure to specify states such as (aq) or (s) in your answers. Submit Answer Retry Entire Group 8 more group attempts remaining Previous Next Save andE +
[Review Topica) [References) Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. When aqueous solutions of sodium fluoride and nitric acid are mixed, an aqueous solution of sodium nitrate and hydrofluoric acid results. Write the net ionic equation for the reaction. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Use H* for the hydronium ion. Be sure to specify states such as (aq) or (s) in your answers. Submit Answer Retry Entire Group 8 more group attempts remaining Previous Next Save andE +
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 58QAP: The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing...
Related questions
Question
Need help pls
Transcribed Image Text:Bookmarks
Window
Help
O O D a
Tue Apr 5 10:"
A cvg.cengagenow.com
+
C OWLV2.
in
D
[Review Toplcs)
References)
Use the References to access important values if needed for this question.
2reg
Be sure to specify states such as (ag) or (s). If a box is not needed leave it blank.
When aqueous solutions of sodium fluoride and nitric acid are mixed, an aqueous solution of sodium nitrate
and hydrofluoric acid results. Write the net ionic equation for the reaction.
2req
Preg
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Use H*
for the hydronium ion. Be sure to specify states such as (aq) or (s) in your answers.
Creq
req
+
+
req
Submit Answer
Retry Entire Group
8 more group attempts remaining
(Previous
Next
Save and Exit
16,354
étv S J
MacBook Air
DII
DD
888
F10
F11
F7
FB
F9
F6
F5
F4
&
24
4.
6
7.
8
Y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
