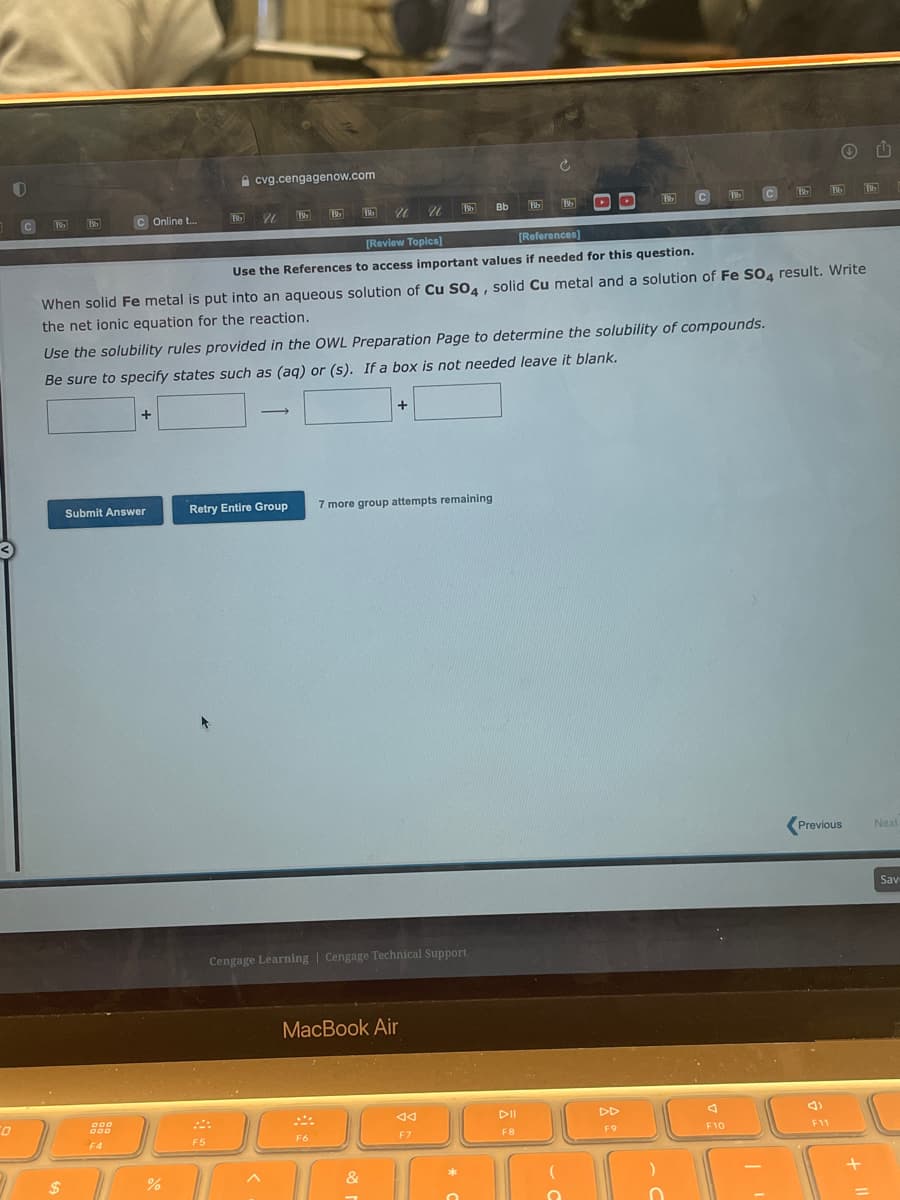
[Review Topica] Use the References to access important values if needed for this question. When solid Fe metal is put into an aqueous solution of Cu So,, solid Cu metal and a solution of Fe SO4 result. Write the net ionic equation for the reaction. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
[Review Topica] Use the References to access important values if needed for this question. When solid Fe metal is put into an aqueous solution of Cu So,, solid Cu metal and a solution of Fe SO4 result. Write the net ionic equation for the reaction. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 142IP
Related questions
Question
Need help please
Transcribed Image Text:A cvg.cengagenow.com
C
C
Bb
C
Bb
C Online t.
[Review Topics)
[References)
Use the References to access important values if needed for this question.
When solid Fe metal is put into an aqueous solution of Cu SO4, solid Cu metal and a solution of Fe SO4 result. Write
the net ionic equation for the reaction.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Submit Answer
Retry Entire Group
7 more group attempts remaining
Previous
Next
Sav
Cengage Learning | Cengage Technical Support
MacBook Air
O00
44
DII
DD
F4
F6
F7
F8
F9
F10
F11
F5
2$
&
*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole