Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.46E: Early attempts to coat metals with Teflon, poly tetrafluoroethylene, resulted in a polymer layer...
Related questions
Question
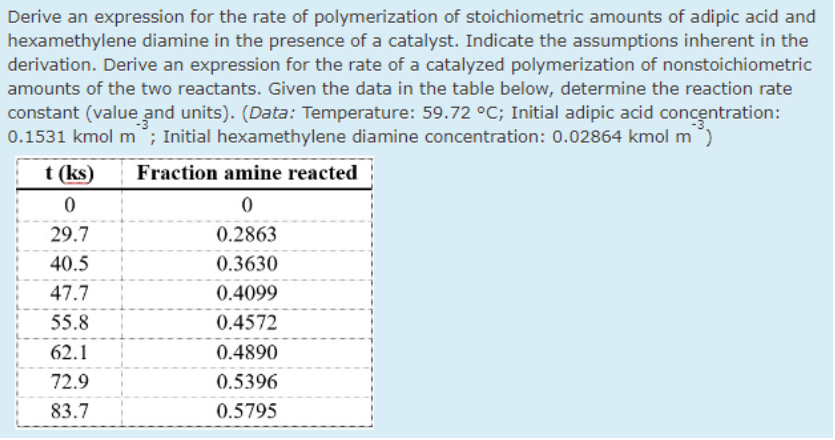
Transcribed Image Text:Derive an expression for the rate of polymerization of stoichiometric amounts of adipic acid and
hexamethylene diamine in the presence of a catalyst. Indicate the assumptions inherent in the
derivation. Derive an expression for the rate of a catalyzed polymerization of nonstoichiometric
amounts of the two reactants. Given the data in the table below, determine the reaction rate
constant (value and units). (Data: Temperature: 59.72 °C; Initial adipic acid concentration:
0.1531 kmol m°; Initial hexamethylene diamine concentration: 0.02864 kmol m)
t (ks)
Fraction amine reacted
29.7
0.2863
40.5
0.3630
47.7
0.4099
55.8
0.4572
62.1
0.4890
72.9
0.5396
83.7
0.5795
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning