rst, a 8.000 g tablet of benzoic acid (C6H5CO₂H) is put into the "bomb" and burned completely in an excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed to rise from 21.00 °C to 62.82 °C over a time of 9.0 minutes. Next, 4.760 g of acetaldehyde (C2H4O) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 21.00 °C to 43.17 °C. Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: -> 2C2H4O(g) + 502(g) → 4CO2(g) + 4H₂O(g) "bomb" chemical reaction A "bomb" calorimeter. Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. water insulation Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match published values for this reaction. Is this reaction exothermic, endothermic, or neither? exothermic endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in the second experiment. OkJ Calculate the reaction enthalpy AHxn per mole of C2H4O. kJ mol J
rst, a 8.000 g tablet of benzoic acid (C6H5CO₂H) is put into the "bomb" and burned completely in an excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed to rise from 21.00 °C to 62.82 °C over a time of 9.0 minutes. Next, 4.760 g of acetaldehyde (C2H4O) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 21.00 °C to 43.17 °C. Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: -> 2C2H4O(g) + 502(g) → 4CO2(g) + 4H₂O(g) "bomb" chemical reaction A "bomb" calorimeter. Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. water insulation Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match published values for this reaction. Is this reaction exothermic, endothermic, or neither? exothermic endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in the second experiment. OkJ Calculate the reaction enthalpy AHxn per mole of C2H4O. kJ mol J
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.103PAE: 9.103 One reason why the energy density of a fuel is important is that to move a vehicle one must...
Related questions
Question
Please don't provide handwriting solutions....
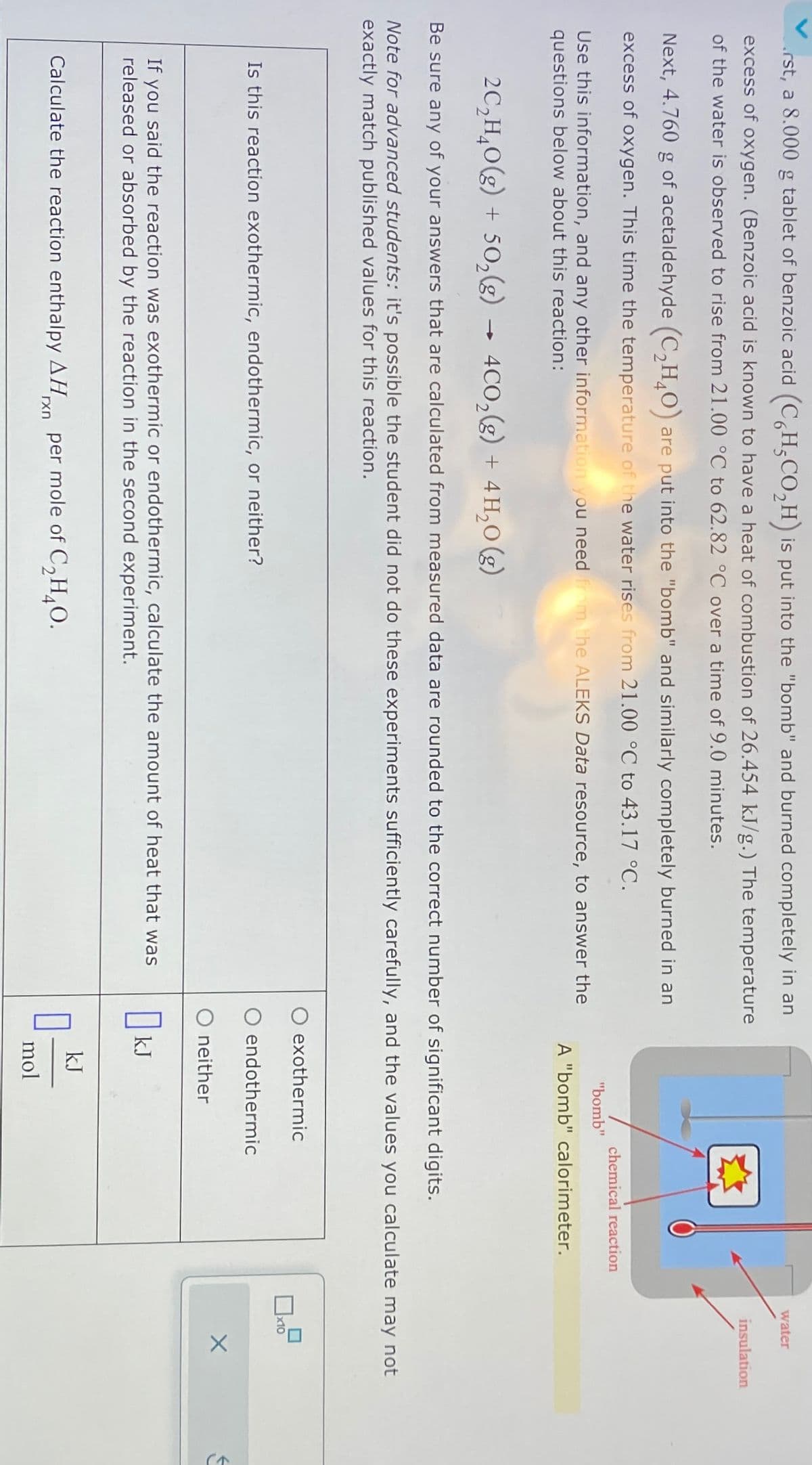
Transcribed Image Text:rst, a 8.000 g tablet of benzoic acid (C6H5CO₂H) is put into the "bomb" and burned completely in an
excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature
of the water is observed to rise from 21.00 °C to 62.82 °C over a time of 9.0 minutes.
Next, 4.760 g of acetaldehyde (C2H4O) are put into the "bomb" and similarly completely burned in an
excess of oxygen. This time the temperature of the water rises from 21.00 °C to 43.17 °C.
Use this information, and any other information you need from the ALEKS Data resource, to answer the
questions below about this reaction:
->
2C2H4O(g) + 502(g) → 4CO2(g) + 4H₂O(g)
"bomb"
chemical reaction
A "bomb" calorimeter.
Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits.
water
insulation
Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not
exactly match published values for this reaction.
Is this reaction exothermic, endothermic, or neither?
exothermic
endothermic
O neither
If you said the reaction was exothermic or endothermic, calculate the amount of heat that was
released or absorbed by the reaction in the second experiment.
OkJ
Calculate the reaction enthalpy AHxn per mole of C2H4O.
kJ
mol
J
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning