Safari File Edit View History Bookmarks Window Help ) 47% Sun 11:03 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H. Review I Constants I Periodic Table Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. Oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. In general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. Part A Which element is oxidized in this reaction? 2CuO + C 2Cu + CO2 Enter the chemical symbol of the element. An oxidizing agent is an element or compound in a redox reaction that oxidizes another species and itself gets reduced and is therefore the electron acceptor in the • View Available Hint(s) reaction. is oxidized A reducing agent is an element or compound in a redox reaction that reduces another species and itself gets oxidized and is therefore the electron donor in the reaction. Submit As a summary, keep in mind the following: • Oxidation means an increase in oxidation state and a loss of electrons and involves a Part B Complete previous part(s) reducing agent. • Reduction means a decrease in oxidation state and a gain of electrons and involves an oxidizing agent. Part C Which element is reduced in this reaction? 2Cr(OH)3 +3OCI- +40H¯→2CrO4 2+3Cl- + 5H2O Enter the chemical symbol of the element. • View Available Hint(s) is reduced Submit FEB 3141503 23 ostv MacBook Pro esc @ 23 & 1 2 3 4 5 8 delete %24 LL
Safari File Edit View History Bookmarks Window Help ) 47% Sun 11:03 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H. Review I Constants I Periodic Table Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. Oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. In general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. Part A Which element is oxidized in this reaction? 2CuO + C 2Cu + CO2 Enter the chemical symbol of the element. An oxidizing agent is an element or compound in a redox reaction that oxidizes another species and itself gets reduced and is therefore the electron acceptor in the • View Available Hint(s) reaction. is oxidized A reducing agent is an element or compound in a redox reaction that reduces another species and itself gets oxidized and is therefore the electron donor in the reaction. Submit As a summary, keep in mind the following: • Oxidation means an increase in oxidation state and a loss of electrons and involves a Part B Complete previous part(s) reducing agent. • Reduction means a decrease in oxidation state and a gain of electrons and involves an oxidizing agent. Part C Which element is reduced in this reaction? 2Cr(OH)3 +3OCI- +40H¯→2CrO4 2+3Cl- + 5H2O Enter the chemical symbol of the element. • View Available Hint(s) is reduced Submit FEB 3141503 23 ostv MacBook Pro esc @ 23 & 1 2 3 4 5 8 delete %24 LL
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Safari
File
Edit View
History Bookmarks
Window Help
) 47%
Sun 11:03 AM
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111...
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H.
<Homework 5
Oxidation-Reduction Reactions
23 of 24
<>
Review I Constants I Periodic Table
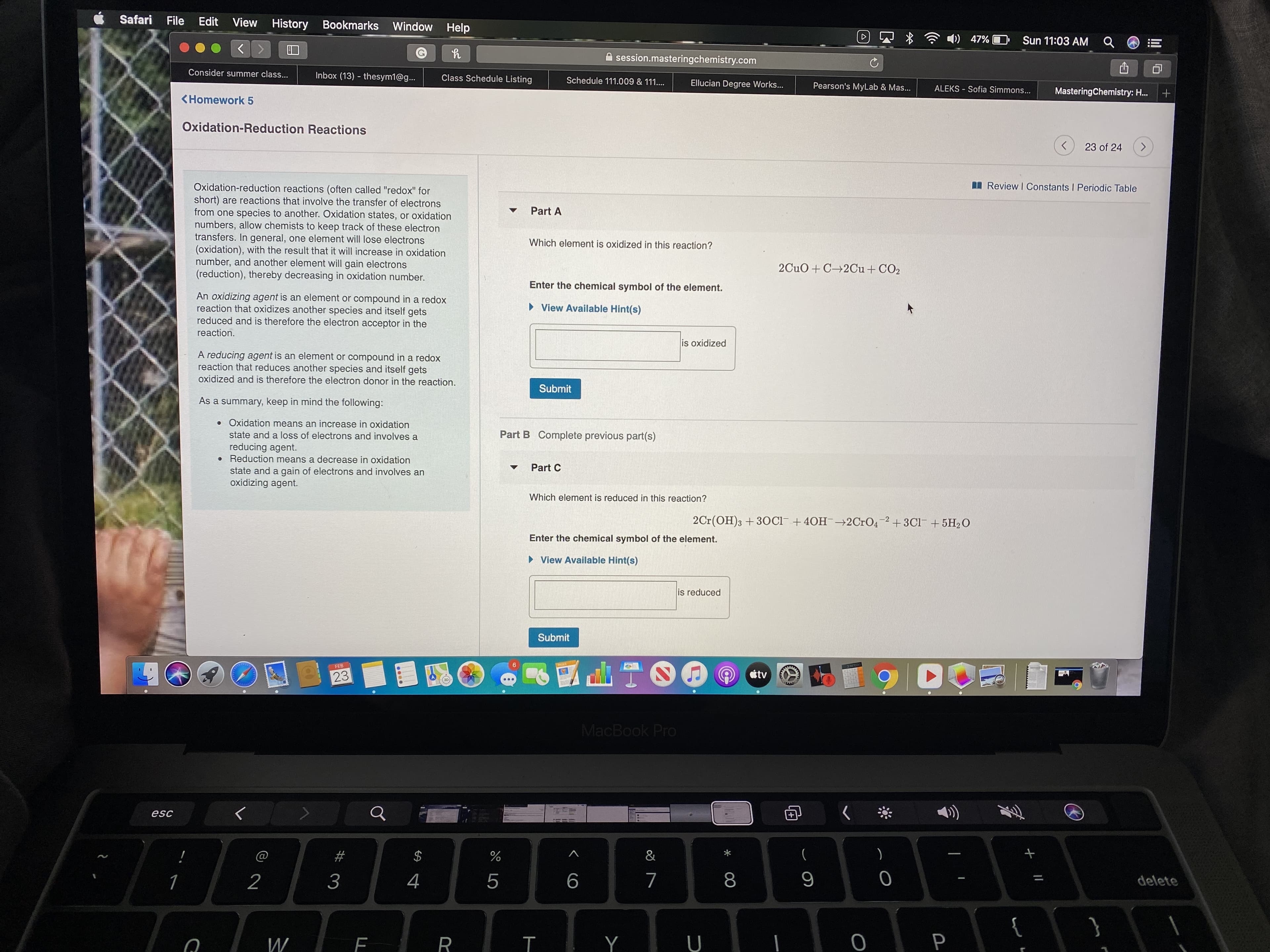
Oxidation-reduction reactions (often called "redox" for
short) are reactions that involve the transfer of electrons
from one species to another. Oxidation states, or oxidation
numbers, allow chemists to keep track of these electron
transfers. In general, one element will lose electrons
(oxidation), with the result that it will increase in oxidation
number, and another element will gain electrons
(reduction), thereby decreasing in oxidation number.
Part A
Which element is oxidized in this reaction?
2CuO + C 2Cu + CO2
Enter the chemical symbol of the element.
An oxidizing agent is an element or compound in a redox
reaction that oxidizes another species and itself gets
reduced and is therefore the electron acceptor in the
• View Available Hint(s)
reaction.
is oxidized
A reducing agent is an element or compound in a redox
reaction that reduces another species and itself gets
oxidized and is therefore the electron donor in the reaction.
Submit
As a summary, keep in mind the following:
• Oxidation means an increase in oxidation
state and a loss of electrons and involves a
Part B Complete previous part(s)
reducing agent.
• Reduction means a decrease in oxidation
state and a gain of electrons and involves an
oxidizing agent.
Part C
Which element is reduced in this reaction?
2Cr(OH)3 +3OCI- +40H¯→2CrO4 2+3Cl- + 5H2O
Enter the chemical symbol of the element.
• View Available Hint(s)
is reduced
Submit
FEB
3141503
23
ostv
MacBook Pro
esc
@
23
&
1
2
3
4
5
8
delete
%24
LL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

