Salt MgCO3 Mg(OH)2 Theoretical Ksp 3.5 x 10-8 1.8 x 10-11 For the standardization of the HCI titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99 g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint. After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein endpoint. Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl₂ required 1.51 mL of the same HCl titrant to titrate a 100.0-ml solution. a. What is the molarity of the HCI titrant? b. Comparing the data in distilled water with the two possible solids, which is the most likely identity of the magnesium salt? Show your full solutions in getting your conclusion. Note: Don't forget to factor in stoichiometry c. What is the average experimental Ksp for both solvents (distilled water and 0.0200 M MgCl2) and individual solubility in each solvent of the magnesium salt? d. What is the percent error in the obtained Ksp value?..
Salt MgCO3 Mg(OH)2 Theoretical Ksp 3.5 x 10-8 1.8 x 10-11 For the standardization of the HCI titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99 g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint. After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein endpoint. Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl₂ required 1.51 mL of the same HCl titrant to titrate a 100.0-ml solution. a. What is the molarity of the HCI titrant? b. Comparing the data in distilled water with the two possible solids, which is the most likely identity of the magnesium salt? Show your full solutions in getting your conclusion. Note: Don't forget to factor in stoichiometry c. What is the average experimental Ksp for both solvents (distilled water and 0.0200 M MgCl2) and individual solubility in each solvent of the magnesium salt? d. What is the percent error in the obtained Ksp value?..
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.20QAP
Related questions
Question
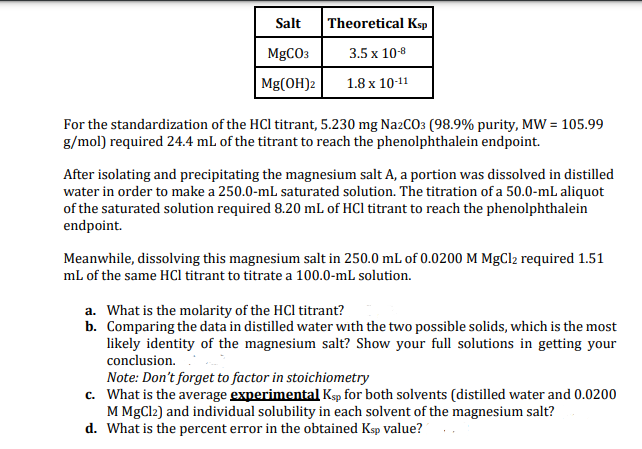
Transcribed Image Text:Salt
MgCO3
Mg(OH)2
Theoretical Ksp
3.5 x 10-8
1.8 x 10-11
For the standardization of the HCI titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99
g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint.
After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled
water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot
of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein
endpoint.
Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl2 required 1.51
mL of the same HCl titrant to titrate a 100.0-mL solution.
a. What is the molarity of the HCI titrant?
b. Comparing the data in distilled water with the two possible solids, which is the most
likely identity of the magnesium salt? Show your full solutions in getting your
conclusion.
Note: Don't forget to factor in stoichiometry
c.
What is the average experimental Ksp for both solvents (distilled water and 0.0200
M MgCl2) and individual solubility in each solvent of the magnesium salt?
d. What is the percent error in the obtained Ksp value?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



