Sample 1 HClag). NaOH(aq). NaHCO3{aq). Water ++ ++ ++ ++ 4. ++ ++ Legend: ++ > completely soluble; +→ slightly soluble; -→ insoluble он он H3C. H2N CH3 он он butane dioic acid cy clohexane butane-2,3-dione cyclopentanamine re sorcinol
Sample 1 HClag). NaOH(aq). NaHCO3{aq). Water ++ ++ ++ ++ 4. ++ ++ Legend: ++ > completely soluble; +→ slightly soluble; -→ insoluble он он H3C. H2N CH3 он он butane dioic acid cy clohexane butane-2,3-dione cyclopentanamine re sorcinol
Chapter20: Carboxylic Acids And Nitriles
Section20.SE: Something Extra
Problem 25MP: Acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of...
Related questions
Question
100%
Professor Acetico Acido has assigned the analysis of unknown organic compound samples to Al Cohol based on their solubility behavior. The summary of the solubility profile are tabulated below along with the possible structures of the samples. Identify each unknown sample by matching the respective solubility profile with the given structures. Briefly explain the reason for your answer.
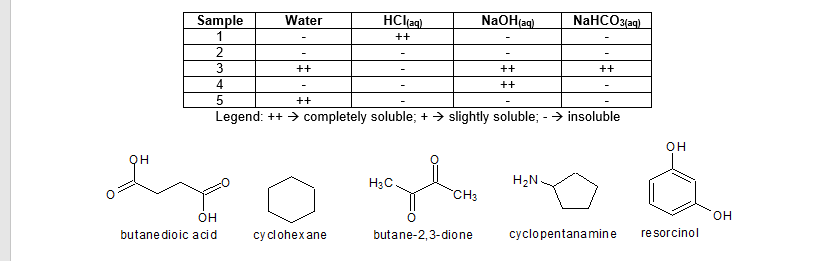
Transcribed Image Text:Sample
Water
HClag)
NaOH(ag)
NaHCO3laq)
1
++
3
++
++
++
4
++
++
Legend: ++ > completely soluble; + > slightly soluble; - > insoluble
он
H3C
H2N.
CH3
он
он
butane dioic acid
cy clohexane
butane-2,3-dione
cyclopentanamine
re sorcinol
Transcribed Image Text:Sample
Identity and Rationalization
2
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

