When 25.0 mL of a 7.08x104 M barium acetate solution is combined with 25.0 mL of a 1.67x10 4 M sodium sulfate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to Solid silver nitrate is slowly added to 125 mL of a 0.0516 M sodium hydroxide solution. The concentration of silver ion required to just initiate precipitation is M.
When 25.0 mL of a 7.08x104 M barium acetate solution is combined with 25.0 mL of a 1.67x10 4 M sodium sulfate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to Solid silver nitrate is slowly added to 125 mL of a 0.0516 M sodium hydroxide solution. The concentration of silver ion required to just initiate precipitation is M.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 30QAP: Silver(I) sulfate (Ksp=1.2105) is used in the electroplating of silver. A 1.0-L solution is prepared...
Related questions
Question
Answer both or please leave for someone else to answerm
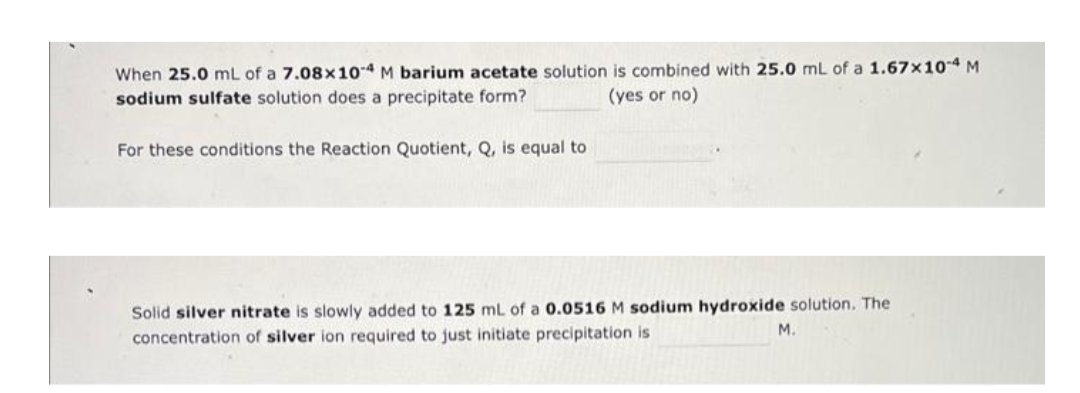
Transcribed Image Text:When 25.0 mL of a 7.08x104 M barium acetate solution is combined with 25.0 mL of a 1.67x10 4 M
sodium sulfate solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
Solid silver nitrate is slowly added to 125 mL of a 0.0516 M sodium hydroxide solution. The
M.
concentration of silver ion required to just initiate precipitation is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning