Sample student prepares three buffer solutions. Each solution is 1.0 M in one of the acids in the table and 1.0 M in its corresponding sodium salt. Which of the solutions has the greatest buffer capacity with respect to added NAOH and why? H. H. CI H-C H-C- H-C C=C 0-H H. 0-H 0-H H. H. Benzoic Acid Chloroacetic Acid Formic Acid Ka pKa 6.2 x 10-5 14 x 10-3 2.86 1.8 x 104 3.75 4.21 The formic acid buffer because it donates both of its hydrogen atoms The chloroacetic acid buffer because itis the strongest acid All are the same The benzoic acd butfer becatse ltis testrongestacid
Sample student prepares three buffer solutions. Each solution is 1.0 M in one of the acids in the table and 1.0 M in its corresponding sodium salt. Which of the solutions has the greatest buffer capacity with respect to added NAOH and why? H. H. CI H-C H-C- H-C C=C 0-H H. 0-H 0-H H. H. Benzoic Acid Chloroacetic Acid Formic Acid Ka pKa 6.2 x 10-5 14 x 10-3 2.86 1.8 x 104 3.75 4.21 The formic acid buffer because it donates both of its hydrogen atoms The chloroacetic acid buffer because itis the strongest acid All are the same The benzoic acd butfer becatse ltis testrongestacid
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.7QAP
Related questions
Question
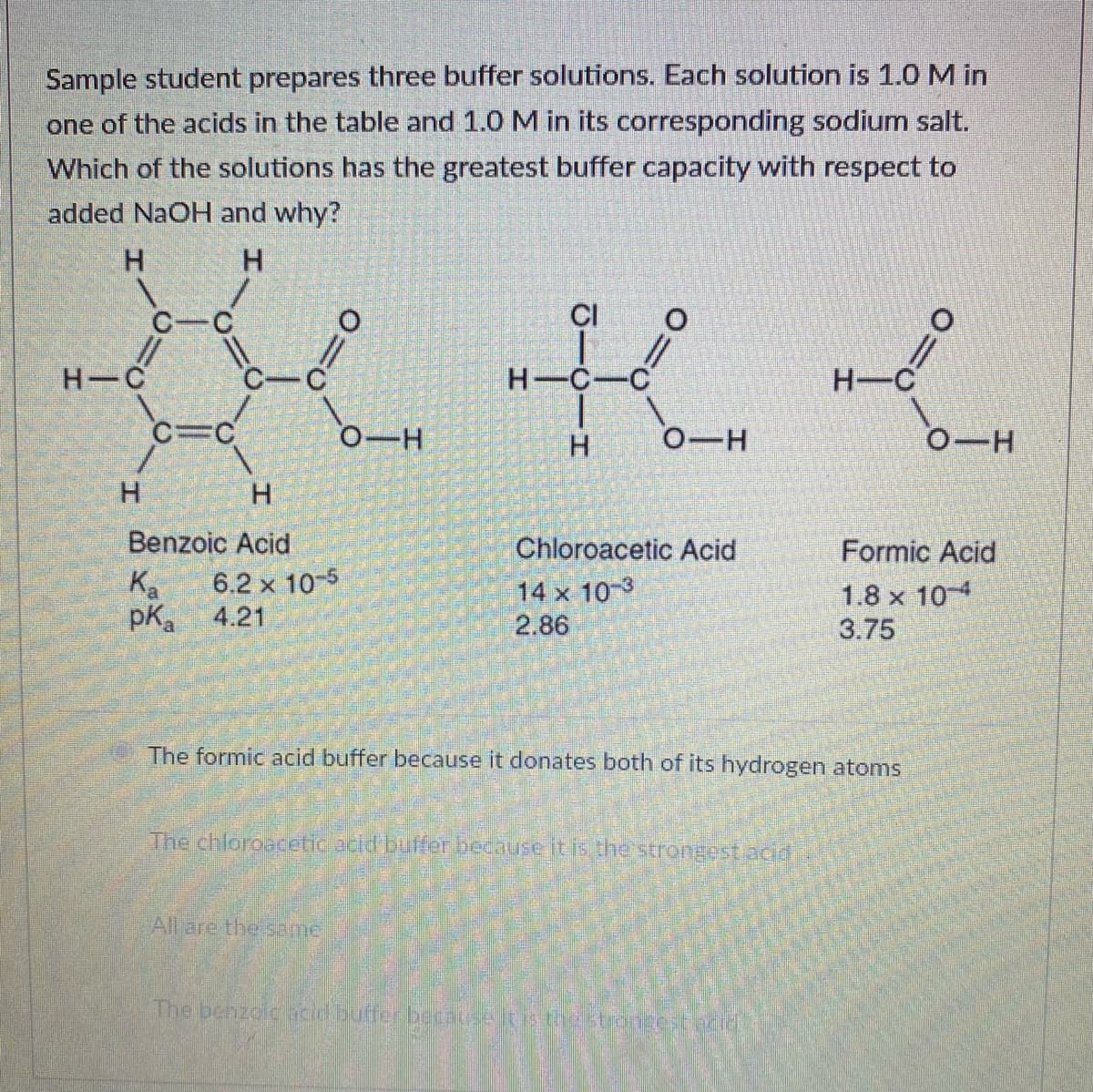
Transcribed Image Text:Sample student prepares three buffer solutions. Each solution is 1.0 M in
one of the acids in the table and 1.0 M in its corresponding sodium salt.
Which of the solutions has the greatest buffer capacity with respect to
added NaOH and why?
H.
H.
CI
H C
H-C-C
H-C
C=C
0-H
H.
0-H
0-H
Benzoic Acid
Chloroacetic Acid
Formic Acid
Ka
pKa
6.2 x 10-5
4.21
14 x 10-3
2.86
1.8 x 10
3.75
The formic acid buffer because it donates both of its hydrogen atoms
The chloroacetic acld buffer becauseitis the strongest acid
All are the same
The benzolc acd butfer because lis testongestecid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning