Saved Rank the following compounds in order of increasing boiling point. OH OH OH Increasing boiling point 1 of 11 Prev Next Delete Insert PrtSc C F5 F12 F10 F11 F8 F9 F7 F6 F4 F3 ( & Backspace 7 6 4 5 3 P T R ST K D F N V V IH LO LL
Saved Rank the following compounds in order of increasing boiling point. OH OH OH Increasing boiling point 1 of 11 Prev Next Delete Insert PrtSc C F5 F12 F10 F11 F8 F9 F7 F6 F4 F3 ( & Backspace 7 6 4 5 3 P T R ST K D F N V V IH LO LL
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter15: Carboxylic Acids And Esters
Section: Chapter Questions
Problem 15.74E
Related questions
Question
100%
Transcribed Image Text:Saved
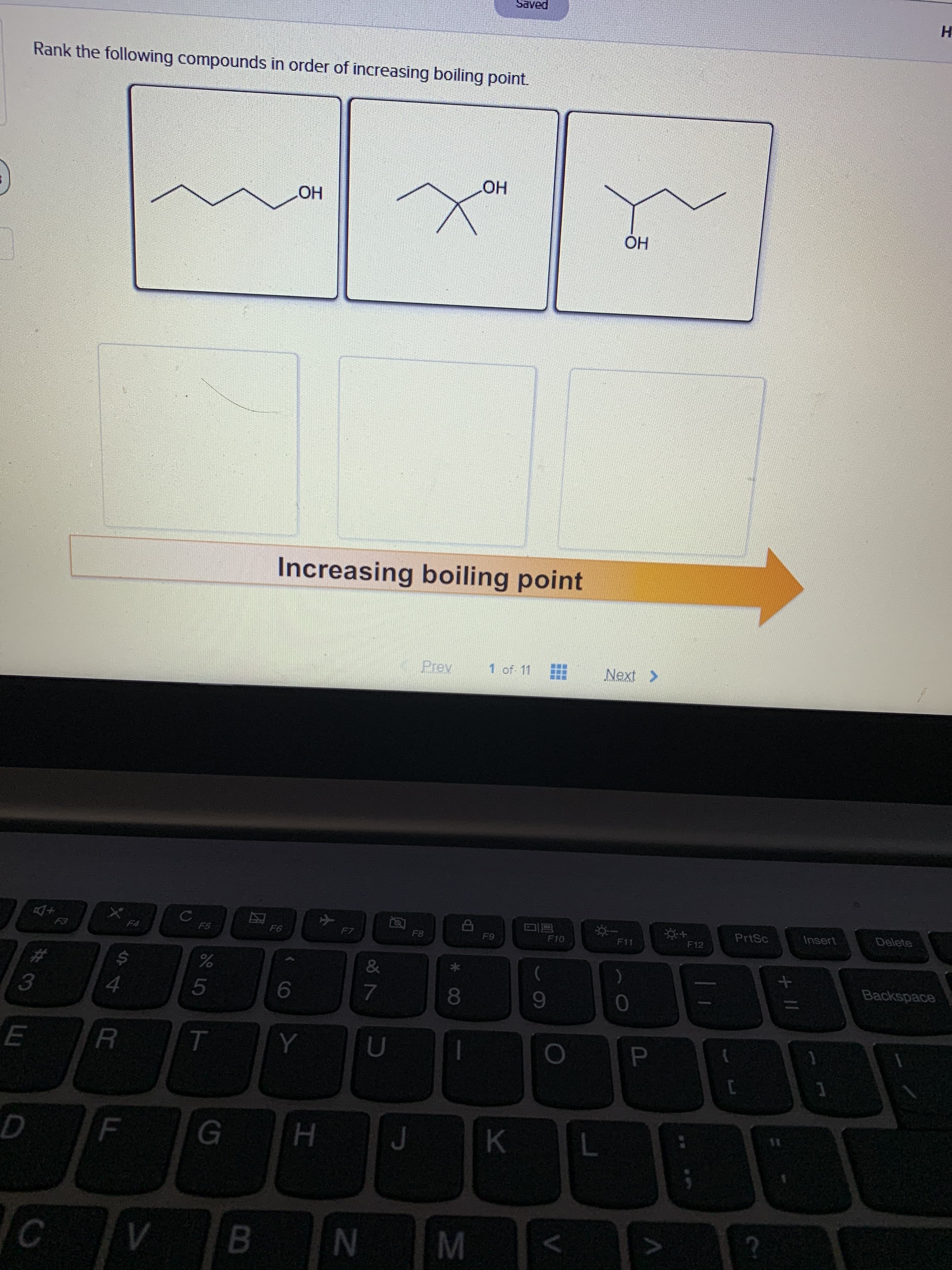
Rank the following compounds in order of increasing boiling point.
OH
OH
OH
Increasing boiling point
1 of 11
Prev
Next
Delete
Insert
PrtSc
C
F5
F12
F10
F11
F8
F9
F7
F6
F4
F3
(
&
Backspace
7
6
4
5
3
P
T
R
ST
K
D
F
N
V
V
IH
LO
LL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning