Science Books presented by Macmillan Leaning Consider the table. Metal Tm (k) All (kJ/mol) T All (kJ/mol) Li 454 2.99 1615 134.7 Na 371 2.60 1156 89.6 K 336 2.33 1033 77.1 Rb 312 2.34 956 69 Cs 302 2.10 942 66 Using the data, calculate AS and AS for Na. 83.4 AS Kmol coet 6.586 AS K-mal munet
Science Books presented by Macmillan Leaning Consider the table. Metal Tm (k) All (kJ/mol) T All (kJ/mol) Li 454 2.99 1615 134.7 Na 371 2.60 1156 89.6 K 336 2.33 1033 77.1 Rb 312 2.34 956 69 Cs 302 2.10 942 66 Using the data, calculate AS and AS for Na. 83.4 AS Kmol coet 6.586 AS K-mal munet
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 7A
Related questions
Question
3
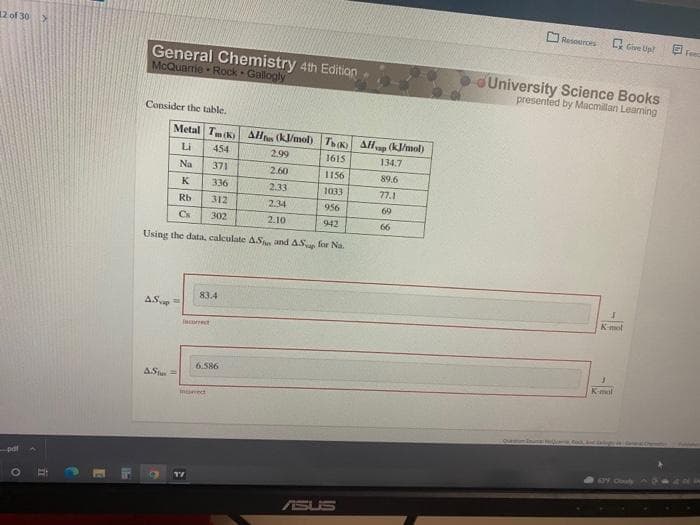
Transcribed Image Text:12 of 30>
O Resouroes
Give Up!
General Chemistry 4th Edition
McQuarie Rock Galogly
University Science Books
presented by Macmillan Leaming
Consider the table.
Metal Tm (K) Al (kJ/mol) TK AH (kJ/mol)
rap
Li
454
2.99
1615
134.7
Na
371
2.60
1156
89.6
K
336
2.33
1033
77.1
Rb
312
2.34
956
60
Cs
302
2.10
942
66
Using the data, calculate ASn and AS for Na.
83.4
AS
Kmol
facorect
6.586
Kmal
ASt=
insect
63Y Obuiy
ASUS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning