SECOND LAW OF THERMODYNAMICS 1. Observe the Figure 1.0 in which the influence of temperature to change in enthalpy, AH and entropy, AS. Write your observations related to the temperature effects on the space provided. AH TAS 5 4 3 1 Figure 1.0 Temperature Effects to Enthalpy and Entropy. Temperature
SECOND LAW OF THERMODYNAMICS 1. Observe the Figure 1.0 in which the influence of temperature to change in enthalpy, AH and entropy, AS. Write your observations related to the temperature effects on the space provided. AH TAS 5 4 3 1 Figure 1.0 Temperature Effects to Enthalpy and Entropy. Temperature
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.69QE: What is the sign of the standard Gibbs free-energy change at low temperatures and at high...
Related questions
Question
100%
Quick overview of our lesson:
Our topic is all about Second Law of
Gibbs’ free energy, G is defined by G = H – TSwhere H is the enthalpy, T is the temperature (in Kelvins), and S is the entropy. In a
please do help me with the questions on the picture/ i think it is to be answered in paragraph form .
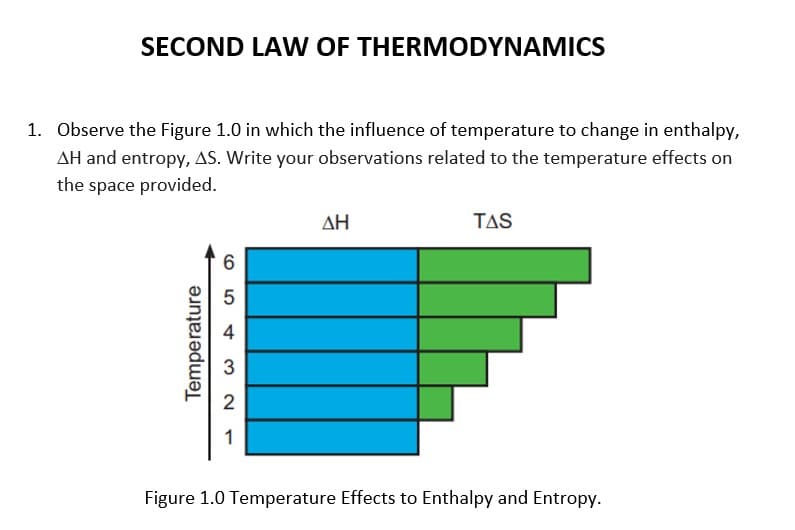
Transcribed Image Text:SECOND LA W OF THERMODYNAMICS
1. Observe the Figure 1.0 in which the influence of temperature to change in enthalpy,
AH and entropy, AS. Write your observations related to the temperature effects on
the space provided.
ΔΗ
TAS
4
3
2
1
Figure 1.0 Temperature Effects to Enthalpy and Entropy.
Temperature
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning