Select all the appropriate balanced nuclear equations for this process. 235 92 235 92 235 92 235 92 38 38 3 235 92 235 92 235 92 235 92 235 92 235 U +_ e → 3_ e +² Pu 94 U → 2₂ He+ Ra 94 U + on → 36 U +on→ U +o n- U + on → +on→ U U 140 55 U 140 54 227 88 140 55 Kr + 139 Ba + 3 n 56 Cs + Xe + Cs + 93 Rb +on 37 Sr+ 2 n Rb + 3 n Ba + n Kr + 94 36 231 He + Th 90 140 +on→ sự Xe t Sr+n U + 1 He 3 He+ Ra 94 38 93 37 139 56 94 38 227 88
Select all the appropriate balanced nuclear equations for this process. 235 92 235 92 235 92 235 92 38 38 3 235 92 235 92 235 92 235 92 235 92 235 U +_ e → 3_ e +² Pu 94 U → 2₂ He+ Ra 94 U + on → 36 U +on→ U +o n- U + on → +on→ U U 140 55 U 140 54 227 88 140 55 Kr + 139 Ba + 3 n 56 Cs + Xe + Cs + 93 Rb +on 37 Sr+ 2 n Rb + 3 n Ba + n Kr + 94 36 231 He + Th 90 140 +on→ sự Xe t Sr+n U + 1 He 3 He+ Ra 94 38 93 37 139 56 94 38 227 88
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 76QRT
Related questions
Question
ANSWER ONLY THE BOTTOM PART
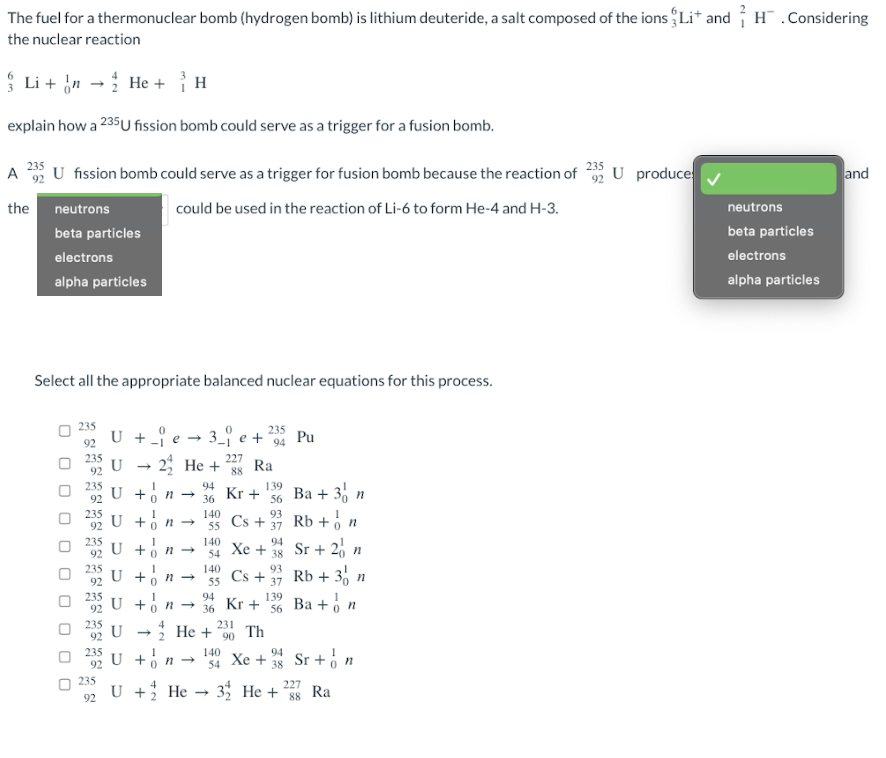
Transcribed Image Text:The fuel for a thermonuclear bomb (hydrogen bomb) is lithium deuteride, a salt composed of the ions Lit and H. Considering
the nuclear reaction
{ Li + 'n → ; He + H
explain how a 235U fission bomb could serve as a trigger for a fusion bomb.
235
A U fission bomb could serve as a trigger for fusion bomb because the reaction of 232 U produce
the
could be used in the reaction of Li-6 to form He-4 and H-3.
neutrons
beta particles
electrons
alpha particles
Select all the appropriate balanced nuclear equations for this process.
235
92
235
92
235
92
235
92
235
92
235
92
235
92
235
92
235
92
235
92
235
U +_je_e+
94
227
U → 2¹ He + Ra
88
Kr +
U + on →
U + on →
0
U
1
U + on →
0
U
1
+ on →
U
1
0
n
94
36
140
55
4
U + 2 He
140
54
140
55
94
36
139
56
Cs +
Xe +
Cs +
Kr +
He + Th
231
90
2
U + on → Xe +
0
93
37
94
38
139
56
93
37
94
38
Pu
Ba + 3⁰ n
Rb +on
Sr + 2 n
Rb + 3 n
Ba + n
140
54
3 He+ Ra
Sr+on
227
88
neutrons
beta particles
electrons
alpha particles
and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning