Select the element whose valence electrons experience a greater effective nuclear charge. Be Why do the valence electrons of the selected element experience a greater effective nuclear charge? The element has more protons and more core electrons than the other element. The element has more protons, but the same number of core electrons as the other element. The element has more core electrons, but fewer protons than the other element. The element has fewer core electrons and more protons than the other element.
Select the element whose valence electrons experience a greater effective nuclear charge. Be Why do the valence electrons of the selected element experience a greater effective nuclear charge? The element has more protons and more core electrons than the other element. The element has more protons, but the same number of core electrons as the other element. The element has more core electrons, but fewer protons than the other element. The element has fewer core electrons and more protons than the other element.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 146AE: An unknown element is a nonmetal and has a valence electron configuration of ns2np4 a. How many...
Related questions
Question
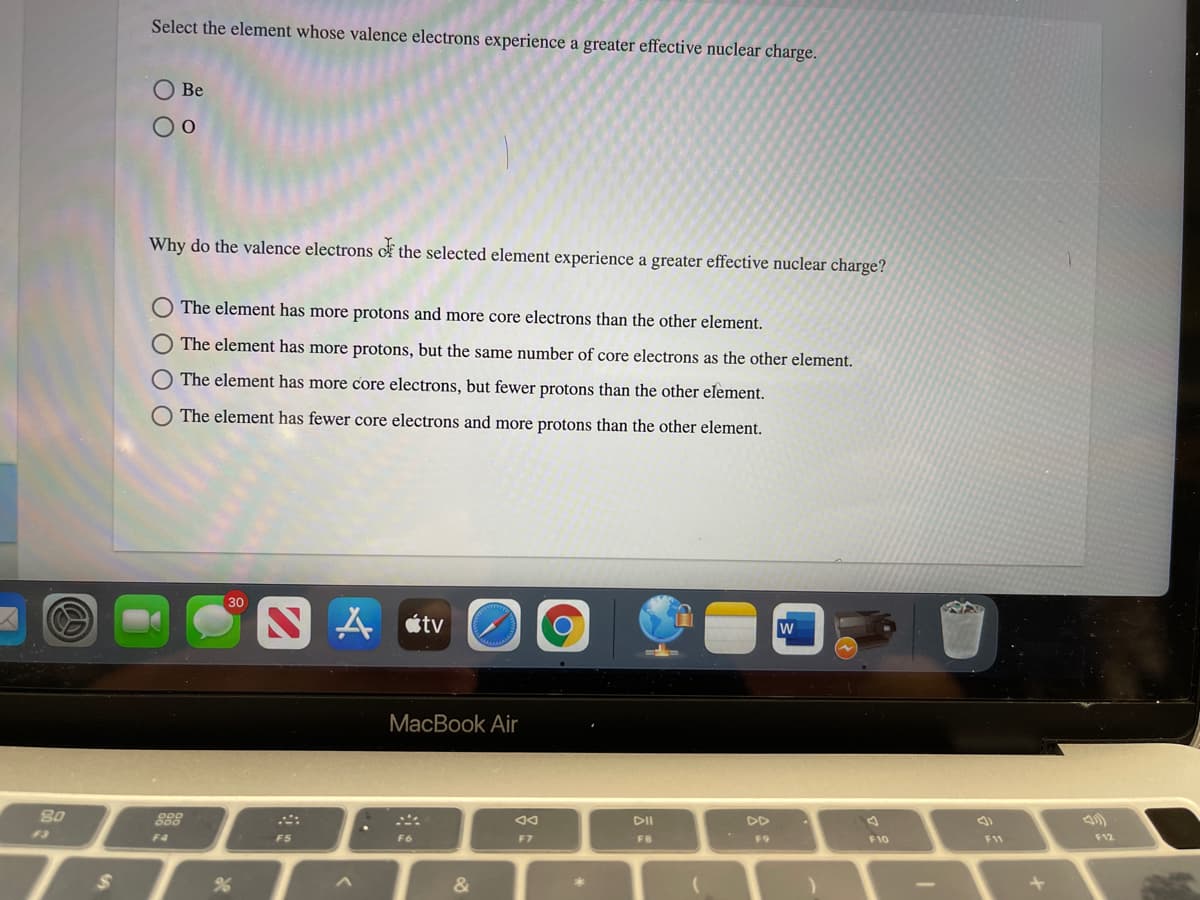
Transcribed Image Text:Select the element whose valence electrons experience a greater effective nuclear charge.
Be
Why do the valence electrons of the selected element experience a greater effective nuclear charge?
The element has more protons and more core electrons than the other element.
The element has more protons, but the same number of core electrons as the other element.
The element has more core electrons, but fewer protons than the other element.
The element has fewer core electrons and more protons than the other element.
30
A étv
w
MacBook Air
80
888
DII
DD
F3
F4
F5
F6
F7
FB
F12
F9
F10
24
&
O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning