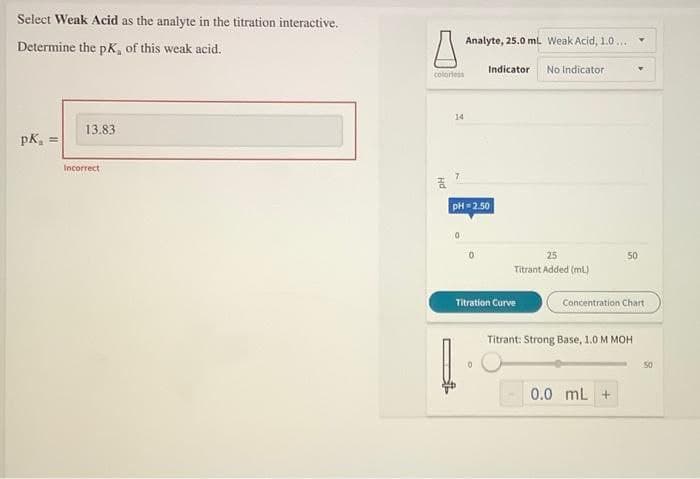
Select Weak Acid as the analyte in the titration interactive. Analyte, 25.0 ml. Weak Acid, 1.0... Determine the pK, of this weak acid. colortess Indicator No Indicator 14 13.83 pK, = Incorrect PH=2.50 25 50 Titrant Added (mL) Titration Curve Concentration Chart Titrant: Strong Base, 1.0 M MOH 50 0.0 ml + Hd
Q: Balance the following in an acidic solution: 103 + SO32-) - 12 + SO42-)
A: Balanced the given redox reaction under acidic solution---
Q: Write the balanced equation for the combustion of Propylene or CH3H6 and Oxygen if propylene is a…
A: Combustion of alkanes gives carbon dioxide gas and water vapors . Enthalpy of combustion can be…
Q: Water is an amphoteric compound. True False
A: Water is amphoteric in Nature because it acts as acid as well as base
Q: Which law of thermodynamics best explains why dissolving ammonium nitrate in water is spontaneous…
A: The first law of thermodynamics describes that the energy of the universe remains constant and it…
Q: The IUPAC name 9s: (CHsCHy)g COH as 2-ethyl pent-3-0l b) 2- ethyl pent-2-01 Cs 2,2-dPethyl but-1-01…
A: Iupac naming is based on rule
Q: Which of the following is/are a Bronsted base? Multiple answers are possible O CO,2 O HBr O NH4 O…
A: Given :- Chemical formula of molecules/ions : CO32- HBr NH4+ H2S HCO3- To identify :-…
Q: Calculate the pH at the following points in a titration of 40.0 mL of 0.100 M barbituric acid (Ka =…
A:
Q: A medical technician is working with the four samples of radionuclides listed in the table below.…
A: We need to arrange the given radioactive isotopes in order of radioactivity
Q: Question 1 What mass of glucose, CH1206, is necessary to prepare 250. milliliters of a 1.00M aqueous…
A: Given-> Volume = 250 ml = 0.250 L (1L =1000 ml) Molarity = 1.00 M
Q: The product of the following reaction L. HeOAc) (CH,COH, THE 2. NaBH OH OH %3D II IV
A:
Q: 1. What is the product for the following reactions? N2OH H20 NaOlf 1,0. A 1. NaH 3. NaB, 0 4. NaOCi,…
A: 1. We have to give the products off the give reactions. P.S.: According to the company…
Q: Give 5 uses of acetone. How is acetone prepared commercially? Show equations. Write the equation for…
A: It is one of the most commonly used solvent moiety in every laboratory. It has several applications.…
Q: A voltaic cell is constructed in which the following cell reaction occurs. The half-cell…
A: 1.) Anode reaction is the oxidation reaction. 2.) Cathode reaction is reduction reaction. 3.)…
Q: The pH scale is a measure of: a. Relative concentration of a solution. b. Relative acidity of…
A: pH is defined as negative logarithm of hydrogen ions dissolved in water.
Q: The correct name for the following struceture Ps: CI- a) Cis-1-chloropent-2-ene oxfde b)…
A:
Q: Predict the product
A:
Q: An overall reaction between nitric oxide and hydrogen is given as follows: 2NO + 2H2 → N2 + 2H2O…
A: To shiw which machanism is consistent ( or both ) we would write rate law expression from both of…
Q: I need help to perdect the ir spectreum for this lab for the staring matrial and the product and…
A:
Q: • Calculate the frequency in Hertz, the energy in Joules, and the energy in electron volts of an…
A: Note : Since you have posted multiple questions, we are entitled to answer the first only. Please…
Q: Balance the following equation and write the sum of the coefficients of the reactants and products…
A:
Q: N. H3 NH3 NH Part A What are the amino acids in kyotorphin? Spell out the full names of the…
A: Name of the amino acids and dipeptide in kyotorphin
Q: Identify the solubility class and give the best solvent that can differentiate the pairs of…
A: A pair of compounds can be separated from each other depending upon their relative polarity on the…
Q: # 3 O no correct response 30. 210 83Bİ→4,He +2063TI + Complete the nuclear equation above. O alpha…
A: Alpha particle - - > It has two protons and two neutrons which are bound to form a particle which…
Q: Determine the molar solubility of AgBr in a solution containing 0.110 mol L-1 NaBr. Ksp (AgBr) = 7.7…
A:
Q: What is the product (s) of the following Reaction? H2 Rh (PPH3),CI r mmr m B A O D&E O C&E O A&B O…
A:
Q: A base that does not gain H + easily is called: a. weak base b. concentrated base c. strong…
A: Define, Brønsted-Lowry theory,
Q: Real life examples of redox reaction
A:
Q: A 0.50-mol sample of oxygen gas is confined at 0 °C and 1.0 atm in a cylinder with a movable piston.…
A: According to the combined gas law, P1V1T1=P2V2T2 Where P1 and P2 are the initial and final…
Q: What is the pH of a solution, having the H30 concentration of 0.0000000028 M? QUESTION 6
A: Given - - > [H3O+] =0.0000000028 M OR [H3O+] = 2.8 x 10-9 M
Q: + а. Bohr b. Dalton C. Rutherford d. Thomson 3. Which atomic model was the first to show a nucleus,…
A: 27) in the Given figure, the model of an atom was proposed by Thomson.
Q: The flame produced by the burner of a gas (propane) grill is a pale blue color when enough air mixes…
A: Given data :
Q: What are the molar concentrations of acetic acid (CH,COOH) and sodium acetate (CH,COONA) in an…
A: Buffer solution: buffer solution is an aqueous solution consisting of a mixture of a weak acid(HA)…
Q: If a sample of a certain solution is determined to have a [H3O+] concentration of 8.94×10^−4…
A:
Q: Describe the solution produced when 5.0 mL 1.0 M CH3COOH is mixed with 3.0 mL 2.5 M hydrochloric…
A: Here in this reaction given acid CH3COOH is a weak acid while HCL is a strong acid acid. So in the…
Q: For pentane, the AH° of vaporization is 26.22 kJ/mol and the AS° of vaporization is 87.88 J/mol·K.…
A:
Q: 6. Classify each of the following equations as direct union, decomposition, single displacement, or…
A:
Q: Tennis balls are usually filled with either air or N2 gas to a pressure above atmospheric pressure…
A: The temperature, pressure, volume, and mole of the gas present in the tennis ball are related by the…
Q: Which of the following ions are always present in an acidic solution? a. hydronium ions b.…
A:
Q: Consider the titration of 25.0 mL of 0.118 M acetic acid (CH, COOH. pK, 4.75) with 0.100 M NaOH. CH,…
A:
Q: When 62.1 g of I2 (253.8 g/mol) reacts with excess hydrogen gas, how many grams of HI (127.9 g/mol)…
A:
Q: Calculate the percentage composition of KMNO,
A: Given :- chemical formula of compound = KMnO4 To determine :- percent composition
Q: Tyler titrates 40 mL of 0.350 M lactic acid (HC3H5O3,Ka=1.5×10−4) using 0.450 M NaOH. Calculate the…
A: 40 mL of 0.350 M lactic acid is tirated using 0.450 M NaOH . Ka of lactic acid = 1.5 × 10-4 .We…
Q: Choose which of the following has the leastSo. metallic Pt methane molten brass (alloy of Cu and…
A: entropy is the randomness in the arrangement of the particles. in solids constituent particles are…
Q: Enumerate several non-toxic organic solvents that can be used to extract the non-polar lipid…
A: 2-propanol / hexane solvent 2-propanol, a quite polar solvent, can denature egg yolk proteins and…
Q: In which of the following aqueous solutions will the Mn(OH)2 be least soluble? Ksp = 2.0x10-13 A.…
A: Adding a common ion decreases the solubility of a component, also known as common ion affect.
Q: What are the speculator ions in 2HCl(aq)+ BaSO4(s)-> BaCl2(aq) + H2SO4(aq)
A:
Q: Glucose (molar mass=180.16 g/mol) is a simple, soluble sugar. Glucose solutions are used to treat…
A: Molar concentration is the ratio of number of moles of solute to volume of solution in liters.…
Q: Identify the strongest acid O CH;OH O H,SO4 O HF О НCN O OH
A: given :- chemical formula of compounds CH3OH H2SO4 HF HCN OH- to identify :- strongest acid
Q: Combine these half-reactions into a balanced overall redox reaction. Zn (s) → Zn2+ (aq) + 2 e Ag"…
A:
Q: Consider the following acids: benzoic acid formic acid hypobromous acid propanoic acid…
A: Given acids are : HC7H5O2 HCO2H HBrO HC3H5O2 6.4×10-5 1.8×10-4 2.8×10-9…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Sources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of the true value (ET) for the quantity being sought by writing either <, >, or = on the space provided TOPIC: Measured mass of the precipitate 1. Filter paper was dried prior to filtration. EX _____ ET TOPIC: Standardization of Titrant 2. Distilled water was not equilibrated to room temperature before the preparation of NaOH titrant. EX ______ ET TOPIC: Determination of Molar Concentration of each component (Double Indicator Titration) 3. No blank correction EX ______ ETSources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of the true value (ET) for the quantity being sought by writing either <, >, or = on the space provided TOPIC: Standardization of Titrant Question 7 Distilled water was not equilibrated to room temperature before the preparation of NaOH titrant. EX __ ET TOPIC: Determination of Molar Concentration of each component (Double Indicator Titration) Question 8 No blank correction Ex ___ ET Question 9 Bubbles trapped in the tip of burette: EX ___ ET Question 10 Measuring the sample volume using a volumetric pipet while looking downwards at the meniscus: EX ___ ETNa2CO3 served as the primary standard in a titration experiment. Find the molarity of the titrant given the following data in 3 decimal places. Show solutions Primary Standard Used: Na2CO3Formula Mass of 1º standard: 105.99 g/mol% purity of 1º standard: 95% Trial 1 2 3 1º Standard weight, g 0.1005 0.1001 0.0997 Net volume of HCl, mL 9.30 9.00 8.90 Molarity of HCl X1 X2 X3
- CrO42- can be used as an indicator when Br- is titrated with Ag+. What concentration of CrO42- should be used so that Ag2CrO4(s) just starts to form at the equivalence point? If a concentration of 0.0010 M CrO42- is used instead, at what concentration of Br- will Ag2CrO(s) just start to precipitate? Do you think this is likely to introduce significant error? The Ksp for Ag2CrO4(s) is 1.2x10-12.A sample is analyzed for chloride by the Volhard method. From the following data, calculate the percentage of chloride present:Weight of sample = 6.0000 g dissolved and diluted to 200 mLAliquot used = 25.00 mL AgNO3 added = 40.00ml of 0.1234MKSCN for back titration = 13.20ml of 0.0930MAdvantages of potentiometric titrations over 'classical' visual indicator methods: (select the correct statement(s)). Can be used for colored, turbid or fluorescent analyte solution. Can be used if there is no suitable indicator or if the color change is difficult to visualize. Both answers 1 and 2 are correct
- While preparing the samples for analysis, you forgot that you already added phenolphthalein indicator so you added some more. What will be its effect in the volume of the titrant needed to reach the endpoint? Select one: A. Increase B. Decrease C. No effect D. Cannot be determined Primary standards are used to determine the exact concentration of the titrant. Based on the criteria of primary standards, which of the following cannot be used as a primary standard? Select one: A.Oxalic acid B. Sodium hydroxide C. Sodium carbonate D. Potassium dichromate During titration, you notice that the retention of the faint pink color is longer than earlier. Your lab partner suggested that you add a “half-drop” to prevent over-titration of the analyte solution. Is it ethical to do the “half-drop” technique? Select one: A. NO. It will cause error in calculations if you forgot to record the volume reading before adding the half-drop. B. YES. No error in calculations will be encountered provided…Given the chemical equation for the anthocyanin extract of red cabbage as an indicator. HIn + H2O ⇌ H3O+ + In- Using the results from the experiment, identify which of the reactant/product are responsible for the red and green color of the indicator.Notes: solution turns red after adding acid; solution turns blue/green after adding base RED: ________________________ BLUE/GREEN: ________________________ Why is the color violet/blue at pH near 7? Answer in 1 sentence. Hint: What is the relationship of the species responsible for the observed colors? __________________________________________________________________please stop rejecting. we pay for this service, so we deserve getting our answer! I do not want to have to put a claim in and report Use acid-base titration to determine theconcentration of:– A strong acid: HCl 0.100 M NaOH (standardized)•0.100 M HCl0.100 M CH3COOH Phenolphthalein is used for endpoint determination– Changes from colourless to pink as a solution becomes more basic• Only need 2-3 drops per titration• pH range: 8.3-10
- A RbOH solution is titrated four (4) times against potassium hydrogen phthalate (KHP; FW=204.224) samples to the Phenolphthalein endpoint. Using the data below, determine the concentration of the RbOH solution? g of KHP Volume of Base Required 0.5373 g 42.49 mL 0.5856 g 43.88 mL 0.5790 g 48.56 mL 0.5856 g 44.60 mL (Report your answer as "mean +/- std dev") M What is the percent relative standard deviation? % What is the 99% Confidence Interval for the concentration of the solution (population mean)?You have performed an iodimetric titration using a commercial vitamin C tablet. Based on the following information below, calculate the %(w/w) of vitamin C(MM=176.16 g/mol) in the tablet: Mass of tablet dissolved in 250.0 mL: 5.422 g Aliquot volume of sample titrated: 25.00 mL Concentration of KIO3: 0.023 M Final burrette volume: 41.31 mL Initial burrette volume: 8.89 mL Blank volume: 0.14 mL2,5 ml volume has taken from an “hypothetic” solution which includes (3+) Sb and (3+) Fe and at the titration with 0.1004 N KMnO4, the wasted amount has found as 16,4 mL. The other 2,5 mL that has taken, has reduced with Zn after that, this 2,5 mL solution has titrates with the same KMnO4 solution solution and the wasted amount is 26,5mL. With these datas find the %concentrations of the ions at the solution.



