Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 34AP
Related questions
Question
Show the structure of the product from the Diels-Alder reaction between 2 moles of isoprene (2-methyl-1,3-butadiene) and one mole of quinone.
Expert Solution
Step 1
Diels-Alder reaction in organic chemistry is a reaction between a conjugated diene and a dinophile (an substituted alkene) to give a cyclohexene derivative product. It is a concerted reaction.
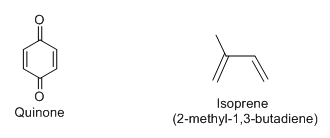
In the given reaction, quinone will be treated as dienophile and isoprene (2-methyl-1,3-butadiene) as conjugated diene.
Structure of quinone and isoprene (2-methyl-1,3-butadiene) is as follows:
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning