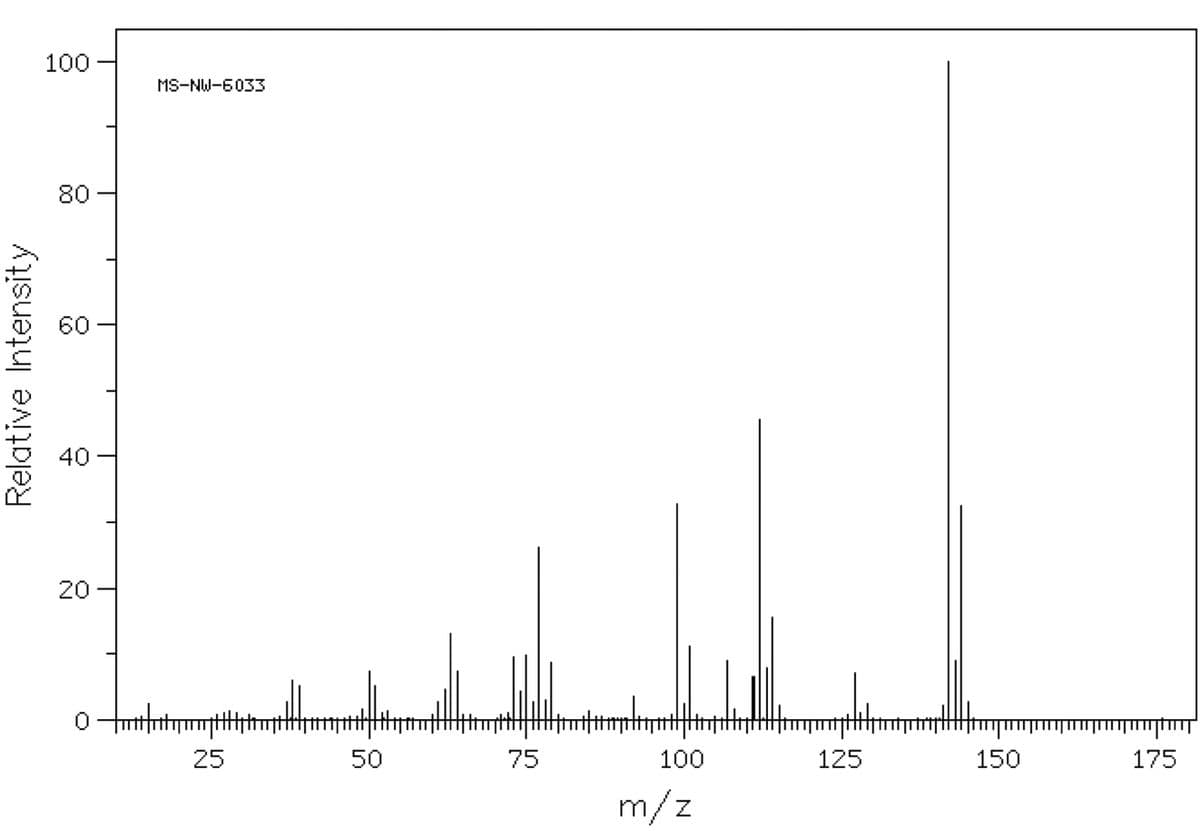
Shown below is the mass spectrum of an aromatic halide. What is the molecular weight of the compound and which halogen is present in the compound?
Q: 2. Indicators are typically weak acids and the equilibrium can be described as follows: HInd+ H₂O…
A: Answer: Le-chatalier's principle suggests that whenever any parameter of the system, that is in…
Q: Draw sketch structures of c6h12 that have the Z-configuratio
A: E–Z configuration is the method of describing the absolute stereochemistry of double bonds. To…
Q: Fill in the blanks in the table. Aqueous solutions are assumed. No justification required. Molality…
A: Given: The weight percentage of MgNH4PO4 in an aqueous solution is 23.0 %. The molality of an…
Q: A + 2 B ⇌ C given the following K values: C ⇌ D + B K1 = 18.06 D ⇌ A + B K2 = 50.62
A:
Q: Q16.2. The ionization constants for sulfurous acid, H₂SO3, at 25°C are Kal=1.3x10-2 and Ka2=6.3x10 .…
A: To calculate the equilibrium concentration of H2SO3, H+, HSO3- and SO32- in a 0.15 M aqueous…
Q: help fill out this sheet based on structure
A:
Q: A certain catalyzed reaction is known to have an activation energy E = 20.0 kJ/mol. Furthermore, the…
A: We will use the Arrhenius equation to find the change in rate when we change the temperature or the…
Q: This graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure,…
A: We are given a vapor pressure curve for three liquids. Gas phase exists above the curve, liquid…
Q: Given the reaction: 2 Na (s) + H₂SO₄ (aq) → Na₂SO₄ (aq) + H₂ (g) What volume of H₂ (in liters) could…
A: The stoichiometric relation in the given balanced chemical equation is moles of H2…
Q: Problem: How many grams of KNO3 would you need to dissolve in 125 g of water to produce a solution…
A: Given -> Weight of water = 125 gm Mole fraction of KNO3 = 0.050
Q: An aqueous solution containing nine millimoles of calcium chloride is mixed with an aqueous solution…
A:
Q: Question 4 How many structural isomers does the molecule below have? The molecule below counts as…
A: Structural isomers are those isomers in which the connectivities of stoms are different and ghe…
Q: CI 1) Mg, diethyl ether 2) i 3) +₂0+
A: -> In presence of Mg/ether alkyl halide can form grignard reagent. -> grignard reagent has…
Q: Draw the most stable and least stable chair confirmations for 1,2,4 - triiodocyclohexane. Which one…
A: Conformational isomers: The conformational isomers are formed by the rotation of a carbon-carbon…
Q: 3. Is the atomic density on the (100) plane of GaAs larger than the atomic density on the (111)…
A:
Q: The drawing below shows a mixture of molecules: carbon nitrogen oxygen key O hydrogen sulfur…
A: It is evident from the drawing that 2 red atoms (oxygen) combine to form a molecule of oxygen while…
Q: The first-order decomposition of N₂O at 1000 K has a rate constant of 0.76 s ¹. If the initial…
A:
Q: The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half…
A:
Q: IR Spectrum d) Wat 4000 100 80 60 41 43 40 10 3000 "C NMR Spectrum (150 MHz, CDC, skon) proton…
A: Given that, the molecular formula of a compound is C5H9N. Also, it's IR, mass, and 1H NMR spectra…
Q: where did the 7.55 come from in step 6?
A: c) Recall the given reaction, Sn2+ aq + Zn s ↔ Sn s + Zn2+ aqEcell…
Q: Home 1- Calculate the hydronium ion concentration of a solution that is 0.080M with respect to ; i.…
A: “Since you have posted multiple questions with multiple sub-parts, we will provide the solution only…
Q: Problem 1.1. Recall the convention from chemistry in labelling atoms: a small letter after a capital…
A: The given unbalanced equations are: a. PbNO32+NaCl→PbCl2+NaNO3. b. C2H2Cl4+CaOH2→C2HCl3+CaCl2+H2O.…
Q: (A) Finish the resonance forms of the sigma complex for the electrophilic aromatic substitution of…
A: An electrophile substitutes an atom that is attached to an aromatic ring in electrophilic aromatic…
Q: Shown below is the major resonance structure for a molecule. Draw the second best resonance…
A: Given : structure of molecule
Q: What is the mechanism of this reaction? H₂PO4
A: -> In presence of acid alcohol give dehydration reaction and form alkene it proceeds through…
Q: 4) Determine if each metal will dissolve in 1M HCl. a) Mg b) Ag c) Mo d) K
A: Using standard reduction potential, we will predict whether metal will dissolve in acid or not.
Q: 0.08, 0.08, 0.09, 0.08 µg/g late the mean, standard deviation, and relative standard deviation of…
A:
Q: Does a reaction occur when aqueous solutions of sodium phosphate and aluminum lodide are combined?…
A:
Q: Arrange these aqueous solutions in order of increasing boiling point. There is no partial credits…
A:
Q: The drawing below shows a mixture of molecules: carbon nitrogen oxygen key O hydrogen sulfur…
A: Limiting reactant: A reactant that is totally consumed when the chemical reaction is completed is…
Q: The structure of sucralose, is shown a) identify the galactose unit and the fructose unit 4)…
A: Aldehyde-containing monosaccharides can cyclize through an intramolecular OH attack on the carbonyl…
Q: If the following reaction is given: C2H5F + Cl2 C2H4Cl2 + HF 151kJ mole || AHran If the bond energy…
A: Given data is The bond energy for C-H is 414 kJ/ mole The bond energy for C-C is 347 kJ/mole The…
Q: The solubility of solid or liquid solutes generally choose your answer... choose your answer... A…
A: The temperature effects the solubility of solid, liquid and gas.
Q: (a) (b) (c) 2-ethylpropylcyclohexane 2,3-diethylhexane 2,3-dimethyl-3-propylpentane
A: Given : incorrect names Tip: first draw structure then write name
Q: Which of the following will always cause an increase in the solubility of a gas in a solvent in…
A: Answer:- This question is answered by using the simple concept of solubility of gases in liquid…
Q: • What are the mayor arganic product of the following reaction What is the stereo chemistry that is…
A:
Q: KBr has a lattice energy of -686 kJ/mol and a hydration energy of -652 kJ/mol. Using this…
A: Answer:- This question is answered by using the simple concept of enthalpy of solution using the…
Q: How can you distinguish the HNMR of N-ethylsaccharin from O-ethylsaccharin? Please explain clearly…
A: We need to describe how we can distinguish the HNMR of N-ethylsaccharin from O-ethylsaccharin.
Q: Order the acids based on their strengths, with the weakest on the left and strongest on the right.…
A: Given data : HCIO - Hypochlorous acid Ka = 3.0 x 10-8 NH3 - Ammonia Kb = 1.8 x 10-5 HCI -…
Q: Please give the major product of the following reactions. You can assume any necessary workup was…
A: Given diols
Q: 9. To isolate benzoic acid, you neutralized "the sodium salt in flask 2". Draw the structure of that…
A: Acid react with base to form salt and water is known as neutralization reaction.
Q: AfH (298 K) = -859 kJ mol-¹, calculate a value for the lattice energy of BaCl₂. Outline any…
A: We have find out the lattice energy of BaCl2.
Q: The harmonic vibrational wavenumber of the 325140 form of sulfur monoxide is 1123.7 cm. Calculate…
A: The force constant of a bond is a direct measure of the interatomic force between the atoms at the…
Q: (e) (g) =O from (c) - ОН CH3MgI H3O+ ether acetyl chloride 0 CI OH O
A: Given : structure of reactant and product
Q: Suppose the boiling point of pure water at high altitude is 85.00 °C. Use the Clausius-Clapeyron…
A:
Q: How many sigma bonds are in a) decane and b) cyclodecane?
A: Since, In the molecule, single bond known as sigma bond. If double bond is present in it contain one…
Q: Cycloalkane Naming Practice
A: Given : structure of molecules
Q: Calculate the amount of heat needed to melt 181. g of solid hexane (C6H₁4) and bring it to a…
A: Given , mass of solid hexane = 181. g
Q: Calculate the amount of heat needed to boil 96.0 g of ethanol (CH₂CH₂OH), beginning from a…
A:
Q: Classify each chemical reaction: Reaction PbCl₂ (aq) + FeSO4 (aq) → FeCl₂(aq) + PbSO4(s) Ca(s) +…
A: In this problem , there are three types of reaction involved : 1) precipitation reaction : When two…
Shown below is the mass spectrum of an
What is the molecular weight of the compound and which halogen is present in the compound?
Step by step
Solved in 2 steps

- Given: A<--> B (ka & ka') B <--> C (kb& kb') so eq 1: d[A]/dt = -ka[A] + ka'[B] eq 2: d[B]/dt= ka[A] - ka'[B] - kb[B] + kb'[C] eq 3: d[C]/dt= kb[B] - kb'[C] Using steady state: ka[A] + kb'[C] = ka'[B] + kb[B] [B]= (ka[A] + kb'[C] ) / (ka' + kb) This can be inserted into equation 3 d[C]/dt = kb[B] - kb'[C] = kb((ka[A] + kb'[C] ) / (ka' + kb)) - kb'[C] Determine the k and k’, where the above mechanism is equivalent to the following: A ↔ C (k and k’) d[A]/dt= -k[A]+ k'[C] d[C]/dt= k[A] - k'[C] What is the simpilfied values of k & k'?5.4.5 Chemical microanalysis5.4.5.1 Exploitation of characteristic X-raysElectron probe microanalysis (EPMA) of bulk samples is now a routine technique for obtaining rapid, accurate analysis of alloys. A small electron probe (100 nm diameter) is used to generate X-rays from a defined area of a polished specimen and the intensity of the various characteristic X-rays measured usingeither wavelength-dispersive spectrometers (WDS) or energy-dispersive spectrometers (EDS). Typically the accuracy of the analysis is š0.1%. One of the limitations of EPMA of bulk samples is that the volume of the sample which contributes to the X-ray signal is relatively independent of the size of theelectron probe, because high-angle elastic scattering of electrons within the sample generates X-rays. The consequence of this is that the spatial resolution of EPMA is no better than ¾2 µm. In the last few years EDX detectors have been interfaced totransmission electron microscopes which are capable of operating…Answer Q45, 46 & 47 show-all-working-explaining-detailly-each-step Answer should be typewritten using a computer keyboard.
- The collision frequency z of a molecule of mass m in a gas at a pressure p is z = 4σ(kT/πm)1/2p/kT, where σ is the collision cross-section. Find an expression for the collision-limited lifetime of an excited state assuming that every collision is effective. Estimate the width of a rotational transition at 63.56 cm−1 in HCl (σ = 0.30 nm2) at 25 °C and 1.0 atm. To what value must the pressure of the gas be reduced in order to ensure that collision broadening is less important than Doppler broadening?An amino acid on the surface of an enzyme was labelled covalently with 1.5-I AEDANS and it is known that the active site contains a tryptophan residue. The fluorescence quantum yield of tryptophan decreased by 15 per cent due to quenching by 1.5-I AEDANS. What is the distance between the active site and the surface of the enzyme?Calculate Ksp for KBr at 25 °C. K+(aq) Br-(aq) KBr(s) ΔG°f (kJ/mol) -283.3 -102.8 -380.7 Provide your answer rounded to 3 significant figures
- Which of the following statements are true about the relationship between C p,m and C v,m ?For 16O2, Δ ᷉ G values for the transitions v = 1 ← 0, 2 ← 0, and 3 ← 0 are, respectively, 1556.22, 3088.28, and 4596.21 cm−1. Calculate ᷉v and xe. Assume ye to be zero.3 1H35Cl has a rotational constant of 10.5 cm-1. Use Excel to plot nJ/n0 from J = 0 to 25 in integer steps at T = 500 K and 1025 K. At each temperature, what J value does (nJ/n0) max occur?
- What is threshold frequency?Consider the quenching of an organic fluorescent species with τ0 = 6.0 ns by a d-metal ion with kQ = 3.0 × 108 dm3 mol−1 s−1. Predict the concentration of quencher required to decrease the fluorescence intensity of the organic species to 50 per cent of the unquenched value.What are the Linear Measurements of J. K. L.

