Problem 1.1. Recall the convention from chemistry in labelling atoms: a small letter after a capital letter belongs to the capital letter, e.g., Pb is a single atom whereas NO3 refers to the molecule consisting of one atom N and three atoms O. For each of the following chemical equations, write down a linear system whose solutions are the amounts of each molecule so that the reaction is balanced. (You don't need to solve the systems.) (a) Pb(NO3)2 + NaCl → PbCl₂ + NaNO3 (b) C₂H₂Cl + Ca(OH)2 → C2HCl3 +CaCl2 + H₂O
Problem 1.1. Recall the convention from chemistry in labelling atoms: a small letter after a capital letter belongs to the capital letter, e.g., Pb is a single atom whereas NO3 refers to the molecule consisting of one atom N and three atoms O. For each of the following chemical equations, write down a linear system whose solutions are the amounts of each molecule so that the reaction is balanced. (You don't need to solve the systems.) (a) Pb(NO3)2 + NaCl → PbCl₂ + NaNO3 (b) C₂H₂Cl + Ca(OH)2 → C2HCl3 +CaCl2 + H₂O
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.50E
Related questions
Question
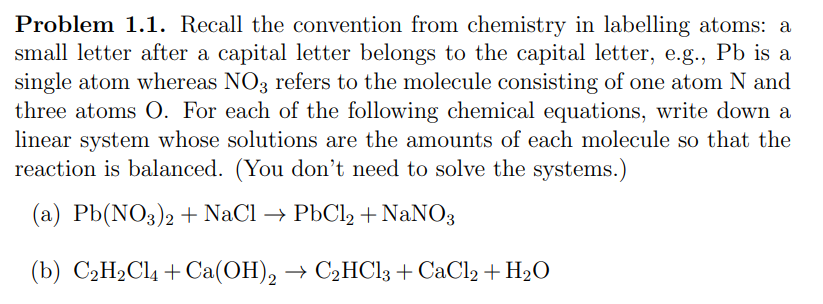
Transcribed Image Text:Problem 1.1. Recall the convention from chemistry in labelling atoms: a
small letter after a capital letter belongs to the capital letter, e.g., Pb is a
single atom whereas NO3 refers to the molecule consisting of one atom N and
three atoms O. For each of the following chemical equations, write down a
linear system whose solutions are the amounts of each molecule so that the
reaction is balanced. (You don't need to solve the systems.)
(a) Pb(NO3)2 + NaCl → PbCl2 + NaNO3
(b) C₂H₂Cl + Ca(OH)2 → C₂HCl3 +CaCl2 + H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning