Silver nitrate reacts with aluminum chloride to form the insoluble compound, silver chloride. The reaction proceeds according to the balanced equation below: 3 AGNO3 (aq) + 1 AICI3 (aq) → 1 Al(NO3), (aq) + 3 AgCI (s) Using the balanced equation above, fill out the rest of the BCA table below by matching the letter with its corresponding molar ratio. Notice that the first row ("Before") has already been done for you. Note: iPad users may have a better viewing experience when their device is in "landscape" orientation. З AgNO3 (аq) AICI3 (aq) Al(NO3)з (аq) 3 AgCI (s) Before 3.00 mol 2.00 mol O mol O mol Change - A mol - B mol + 1.00 mol + C mol After D mol E mol 1.00 mol F mol
Silver nitrate reacts with aluminum chloride to form the insoluble compound, silver chloride. The reaction proceeds according to the balanced equation below: 3 AGNO3 (aq) + 1 AICI3 (aq) → 1 Al(NO3), (aq) + 3 AgCI (s) Using the balanced equation above, fill out the rest of the BCA table below by matching the letter with its corresponding molar ratio. Notice that the first row ("Before") has already been done for you. Note: iPad users may have a better viewing experience when their device is in "landscape" orientation. З AgNO3 (аq) AICI3 (aq) Al(NO3)з (аq) 3 AgCI (s) Before 3.00 mol 2.00 mol O mol O mol Change - A mol - B mol + 1.00 mol + C mol After D mol E mol 1.00 mol F mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 108QRT
Related questions
Question
100%
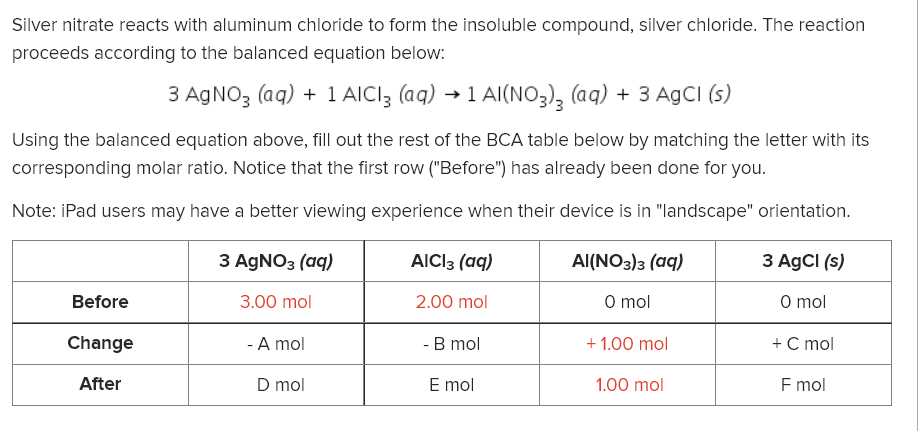
Transcribed Image Text:Silver nitrate reacts with aluminum chloride to form the insoluble compound, silver chloride. The reaction
proceeds according to the balanced equation below:
3 AGNO3 (aq) + 1 AICI3 (aq) → 1 Al(NO3), (aq) + 3 AgCI (s)
Using the balanced equation above, fill out the rest of the BCA table below by matching the letter with its
corresponding molar ratio. Notice that the first row ("Before") has already been done for you.
Note: iPad users may have a better viewing experience when their device is in "landscape" orientation.
З AgNO3 (аq)
AICI3 (aq)
Al(NO3)з (аq)
3 AgCI (s)
Before
3.00 mol
2.00 mol
O mol
O mol
Change
- A mol
- B mol
+ 1.00 mol
+ C mol
After
D mol
E mol
1.00 mol
F mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning